Page 142 - Introduction to chemical reaction engineering and kinetics
P. 142
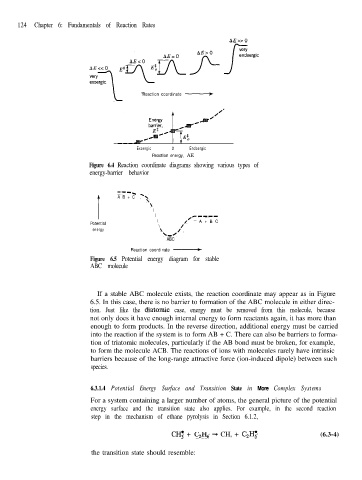
124 Chapter 6: Fundamentals of Reaction Rates
AE>>O
Reaction coordinate -
Exoergic 0 Endoergic
Reaction energy, AE
Figure 6.4 Reaction coordinate diagrams showing various types of
energy-barrier behavior
----
A B + C -\
\
\
\
\ -mm-
\ ‘+- A + B C
Potential \ /
energy \ /’
k-R
ABC
Reaction coordinate -
Figure 6.5 Potential energy diagram for stable
ABC molecule
If a stable ABC molecule exists, the reaction coordinate may appear as in Figure
6.5. In this case, there is no barrier to formation of the ABC molecule in either direc-
tion. Just like the diatomic case, energy must be removed from this molecule, because
not only does it have enough internal energy to form reactants again, it has more than
enough to form products. In the reverse direction, additional energy must be carried
into the reaction if the system is to form AB + C. There can also be barriers to forma-
tion of triatomic molecules, particularly if the AB bond must be broken, for example,
to form the molecule ACB. The reactions of ions with molecules rarely have intrinsic
barriers because of the long-range attractive force (ion-induced dipole) between such
species.
6.3.1.4 Potential Energy Surface and Transition State in More Complex Systems
For a system containing a larger number of atoms, the general picture of the potential
energy surface and the transition state also applies. For example, in the second reaction
step in the mechanism of ethane pyrolysis in Section 6.1.2,
CHj + GH, -+ CH, + C2H; (6.3-4)
the transition state should resemble: