Page 323 - Introduction to chemical reaction engineering and kinetics
P. 323
304 Chapter 12: Batch Reactors (BR)
T,IK
tlmin
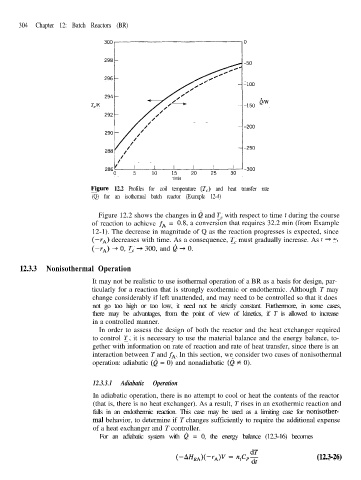
Figure 12.2 Profiles for coil temperature (T,) and heat transfer rate
(Q) for an isothermal batch reactor (Example 12-4)
Figure 12.2 shows the changes in Q and T, with respect to time t during the course
of reaction to achieve fA = 0.8, a conversion that requires 32.2 min (from Example
12-1). The decrease in magnitude of Q as the reaction progresses is expected, since
(--I*) decreases with time. As a consequence, T, must gradually increase. As t 4 00,
(-T*) -+ 0, T, + 300, and Q + 0.
12.3.3 Nonisothermal Operation
It may not be realistic to use isothermal operation of a BR as a basis for design, par-
ticularly for a reaction that is strongly exothermic or endothermic. Although T may
change considerably if left unattended, and may need to be controlled so that it does
not go too high or too low, it need not be strictly constant. Furthermore, in some cases,
there may be advantages, from the point of view of kinetics, if T is allowed to increase
in a controlled manner.
In order to assess the design of both the reactor and the heat exchanger required
to control T, it is necessary to use the material balance and the energy balance, to-
gether with information on rate of reaction and rate of heat transfer, since there is an
interaction between T and fA. In this section, we consider two cases of nonisothermal
operation: adiabatic (Q = 0) and nonadiabatic (Q # 0).
12.3.3.1 Adiabatic Operation
In adiabatic operation, there is no attempt to cool or heat the contents of the reactor
(that is, there is no heat exchanger). As a result, T rises in an exothermic reaction and
falls in an endothermic reaction. This case may be used as a limiting case for nonisother-
ma1 behavior, to determine if T changes sufficiently to require the additional expense
of a heat exchanger and T controller.
For an adiabatic system with Q = 0, the energy balance (12.3-16) becomes
(12.3-26)