Page 36 - Introduction to chemical reaction engineering and kinetics
P. 36
18 Chapter 1: Introduction
1.54 An Example of an Industrial Reactor
One of the most important industrial chemical processes is the manufacture of sulfuric
acid. A major step in this process is the oxidation of SO, with air or oxygen-enriched air
in the reversible, exothermic reaction corresponding to equation (A) in Example 1-2:
so, + ;oz 2 so,
This is carried out in a continuous-flow reactor (“SO, converter”) in several stages, each
stage containing a bed of particles of catalyst (promoted V,O,).
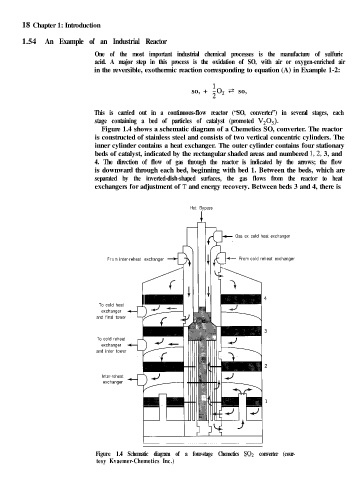
Figure 1.4 shows a schematic diagram of a Chemetics SO, converter. The reactor
is constructed of stainless steel and consists of two vertical concentric cylinders. The
inner cylinder contains a heat exchanger. The outer cylinder contains four stationary
beds of catalyst, indicated by the rectangular shaded areas and numbered 1,2, 3, and
4. The direction of flow of gas through the reactor is indicated by the arrows; the flow
is downward through each bed, beginning with bed 1. Between the beds, which are
separated by the inverted-dish-shaped surfaces, the gas flows from the reactor to heat
exchangers for adjustment of T and energy recovery. Between beds 3 and 4, there is
Hot Bypass
(a- Gas ex cold heat exchanger
F r o m inter-reheat exchanger From cold reheat exchanger
To cold heat J
exchanger
and final tower
To cold reheat
exchanger
and inter tower
Inter-reheat
exchanger
I I
Figure 1.4 Schematic diagram of a four-stage Chemetics SO2 converter (cour-
tesy Kvaemer-Chemetics Inc.)