Page 37 - Introduction to chemical reaction engineering and kinetics
P. 37
1.6 Dimensions and Units 19
also flow through an “inter tower” for partial absorption of SO, (to form acid). The
gas from bed 4 flows to a “final tower” for complete absorption of S03. During passage
of reacting gas through the beds, the reaction occurs adiabatically, and hence T rises.
The operating temperature range for the catalyst is about 400°C to 600°C. The catalyst
particles contain a few percent of the active ingredients, and are either cylindrical or
ringlike in shape, with dimensions of a few mm. From economic and environmental
(low SO,-emission) considerations, the fractional conversion of SO, should be as high
as possible, and can be greater than 99%.
Some important process design and operating questions for this reactor are:
(1) Why is the catalyst arranged in four shallow beds rather than in one deeper bed?
(2) What determines the amount of catalyst required in each bed (for a given plant
capacity)? How is the amount calculated?
(3) What determines the depth and diameter of each bed? How are they calculated?
(4) What determines the temperature of the gas entering and leaving each stage?
The answers to these questions are contained in part in the reversible, exothermic
nature of the reaction, in the adiabatic mode of operation, and in the characteristics of
the catalyst. We explore these issues further in Chapters 5 and 21.
1.6 DIMENSIONS AND UNITS
For the most part, in this book we use SI dimensions and units (SI stands for Ze systdme
international d’uniti%). A dimension is a name given to a measurable quantity (e.g.,
length), and a unit is a standard measure of a dimension (e.g., meter (for length)). SI
specifies certain quantities as primary dimensions, together with their units. A primary
dimension is one of a set, the members of which, in an absolute system, cannot be related
to each other by definitions or laws. All other dimensions are secondary, and each can
be related to the primary dimensions by a dimensional formula. The choice of primary
dimensions is, to a certain extent, arbitrary, but their minimum number, determined
as a matter of experience, is not. The number of primary dimensions chosen may be
increased above the minimum number, but for each one added, a dimensional constant
is required to relate two (or more) of them.
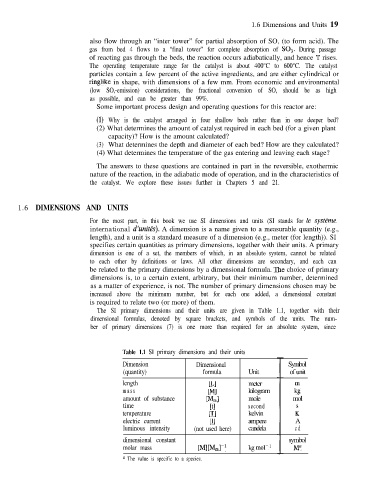
The SI primary dimensions and their units are given in Table 1.1, together with their
dimensional formulas, denoted by square brackets, and symbols of the units. The num-
ber of primary dimensions (7) is one more than required for an absolute system, since
Table 1.1 SI primary dimensions and their units
Dimension Dimensional Symbol
(quantity) formula Unit of unit
length [Ll meter
mass WI kilogram G
amount of substance P&l mole mol
time rt1 second
temperature PI kelvin Ii
electric current [II ampere A
luminous intensity (not used here) candela cd
dimensional constant symbol
molar mass PflD4J’ kg mol- ’ Ma
a The value is specific to a species.