Page 23 - Laboratory Manual in Physical Geology
P. 23
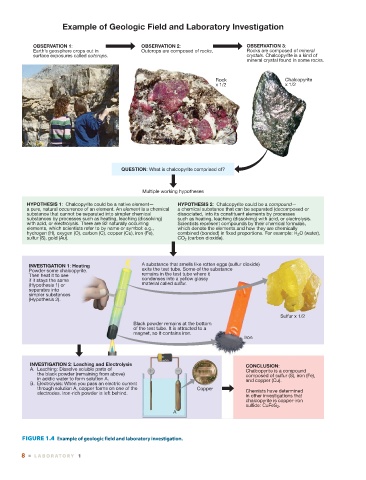
Example of Geologic Field and Laboratory Investigation
OBSERVATION 1: OBSERVATION 2: OBSERVATION 3:
Earth’s geosphere crops out in Outcrops are composed of rocks. Rocks are composed of mineral
surface exposures called outcrops. crystals. Chalcopyrite is a kind of
mineral crystal found in some rocks.
Rock Chalcopyrite
x 1/2 x 1/2
QUESTION: What is chalcopyrite comprised of?
Multiple working hypotheses
HYPOTHESIS 1: Chalcopyrite could be a native element— HYPOTHESIS 2: Chalcopyrite could be a compound—
a pure, natural occurrence of an element. An element is a chemical a chemical substance that can be separated (decomposed or
substance that cannot be separated into simpler chemical dissociated, into its constituent elements by processes
substances by processes such as heating, leaching (dissolving) such as heating, leaching (dissolving) with acid, or electrolysis.
with acid, or electrolysis. There are 92 naturally occurring Scientists represent compounds by their chemical formulas,
elements, which scientists refer to by name or symbol: e.g., which denote the elements and how they are chemically
hydrogen (H), oxygen (O), carbon (C), copper (Cu), iron (Fe), combined (bonded) in fixed proportions. For example: H 2 O (water),
sulfur (S), gold (Au). CO 2 (carbon dioxide).
INVESTIGATION 1: Heating A substance that smells like rotten eggs (sulfur dioxide)
Powder some chalcopyrite. exits the test tube. Some of the substance
Then heat it to see remains in the test tube where it
if it stays the same condenses into a yellow glassy
(Hypothesis 1) or material called sulfur.
separates into
simpler substances
(Hypothesis 2).
Sulfur x 1/2
Black powder remains at the bottom
of the test tube. It is attracted to a
magnet, so it contains iron.
Iron
INVESTIGATION 2: Leaching and Electrolysis CONCLUSION:
A. Leaching: Dissolve soluble parts of Chalcopyrite is a compound
the black powder (remaining from above) composed of sulfur (S), iron (Fe),
in acidic water to form solution A. and copper (Cu).
B. Electrolysis: When you pass an electric current
through solution A, copper forms on one of the Copper Chemists have determined
electrodes. Iron-rich powder is left behind.
in other investigations that
chalcopyrite is copper-iron
sulfide: CuFeS 2 .
A
FIGURE 1.4 Example of geologic field and laboratory investigation.
8 ■ L ABOR ATORY 1