Page 217 - Lindens Handbook of Batteries
P. 217
ZINC-CARBON BATTERIES—LECLANCHÉ AND ZINC CHLORIDE CELL SYSTEMS 9.11
water). The powdered carbon serves the dual purpose of adding electrical conductivity to the MnO ,
2
which itself has high electrical resistance. It also acts as a means of holding the electrolyte. The
cathode mixing and forming processes are also important since they determine the homogeneity of
the cathode mix and the compaction characteristics associated with the different methods of manu-
facture. This becomes more critical in the case of the zinc chloride cell, where the cathode contains
proportions of liquid that range between 60 and 75% by volume.
Of the various forming methods available, mix extrusion and compaction-then-insertion are the
two used most widely. On the other hand, there is a wide variety of techniques for mixing. The most
popular methods are cement-style mixers, mash mixers, and rotary mullor mixers. Both techniques
offer the ability to manufacture large quantities of mix in relatively short times and minimize the
shearing effect upon the carbon black, which reduces its ability to hold solution. The bobbin usually
contains ratios of manganese dioxide to powdered carbon from 3:1 to as much as 11:1 by weight.
Also, 1:1 ratios have been used in batteries for photoflash applications where high pulses of current
are more important than capacity.
9.5.3 Manganese Dioxide (MnO )
2
The types of manganese dioxide used in dry cells are generally categorized as natural manganese
dioxide (NMD), activated manganese dioxide (AMD), chemically synthesized manganese diox-
ide (CMD), and electrolytically deposited manganese dioxide (EMD). EMD is a more expensive
material that has a gamma-phase crystal structure. CMD has a delta-phase structure, and NMDs
have the alpha, beta, and gamma phases of MnO . EMD, while more expensive, results in a higher
2
cell capacity with improved rate capability and is used in heavy or industrial applications. As
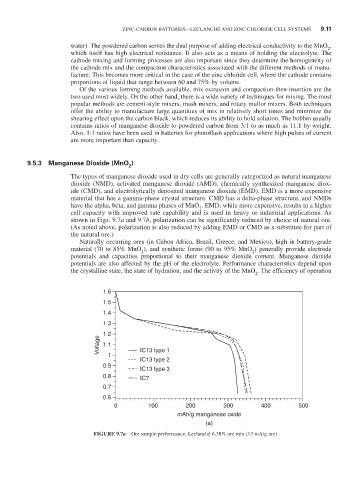
shown in Figs. 9.7a and 9.7b, polarization can be significantly reduced by choice of natural ore.
(As noted above, polarization is also reduced by adding EMD or CMD as a substitute for part of
the natural ore.)
Naturally occurring ores (in Gabon Africa, Brazil, Greece, and Mexico), high in battery-grade
material (70 to 85% MnO ), and synthetic forms (90 to 95% MnO ) generally provide electrode
2
2
potentials and capacities proportional to their manganese dioxide content. Manganese dioxide
potentials are also affected by the pH of the electrolyte. Performance characteristics depend upon
the crystalline state, the state of hydration, and the activity of the MnO . The efficiency of operation
2
1.6
1.5
1.4
1.3
1.2
Voltage 1.1 IC13 type 1
1
IC13 type 2
0.9
IC13 type 3
0.8 IC7
0.7
0.6
0 100 200 300 400 500
mAh/g manganese oxide
(a)
FIGURE 9.7a Ore sample performance, Leclanché 6.38% ore mix (13 mA/g ore)