Page 222 - Lindens Handbook of Batteries
P. 222
9.16 PRIMARY BATTERIES
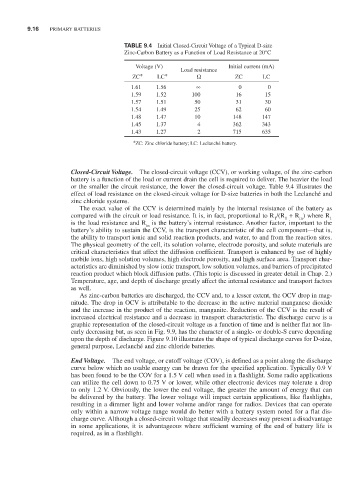
TABLE 9.4 Initial Closed-Circuit Voltage of a Typical D-size
Zinc-Carbon Battery as a Function of Load Resistance at 20°C
Voltage (V) Initial current (mA)
Load resistance
ZC* LC* Ω ZC LC
1.61 1.56 ∞ 0 0
1.59 1.52 100 16 15
1.57 1.51 50 31 30
1.54 1.49 25 62 60
1.48 1.47 10 148 147
1.45 1.37 4 362 343
1.43 1.27 2 715 635
*ZC: Zinc chloride battery; LC: Leclanché battery.
Closed-Circuit Voltage. The closed-circuit voltage (CCV), or working voltage, of the zinc-carbon
battery is a function of the load or current drain the cell is required to deliver. The heavier the load
or the smaller the circuit resistance, the lower the closed-circuit voltage. Table 9.4 illustrates the
effect of load resistance on the closed-circuit voltage for D-size batteries in both the Leclanché and
zinc chloride systems.
The exact value of the CCV is determined mainly by the internal resistance of the battery as
compared with the circuit or load resistance. It is, in fact, proportional to R /(R + R ) where R
in
1
1
1
is the load resistance and R is the battery’s internal resistance. Another factor, important to the
in
battery’s ability to sustain the CCV, is the transport characteristic of the cell component—that is,
the ability to transport ionic and solid reaction products, and water, to and from the reaction sites.
The physical geometry of the cell, its solution volume, electrode porosity, and solute materials are
critical characteristics that affect the diffusion coefficient. Transport is enhanced by use of highly
mobile ions, high solution volumes, high electrode porosity, and high surface area. Transport char-
acteristics are diminished by slow ionic transport, low solution volumes, and barriers of precipitated
reaction product which block diffusion paths. (This topic is discussed in greater detail in Chap. 2.)
Temperature, age, and depth of discharge greatly affect the internal resistance and transport factors
as well.
As zinc-carbon batteries are discharged, the CCV and, to a lesser extent, the OCV drop in mag-
nitude. The drop in OCV is attributable to the decrease in the active material manganese dioxide
and the increase in the product of the reaction, manganite. Reduction of the CCV is the result of
increased electrical resistance and a decrease in transport characteristic. The discharge curve is a
graphic representation of the closed-circuit voltage as a function of time and is neither flat nor lin-
early decreasing but, as seen in Fig. 9.9, has the character of a single- or double-S curve depending
upon the depth of discharge. Figure 9.10 illustrates the shape of typical discharge curves for D-size,
general purpose, Leclanché and zinc chloride batteries.
End Voltage. The end voltage, or cutoff voltage (COV), is defined as a point along the discharge
curve below which no usable energy can be drawn for the specified application. Typically 0.9 V
has been found to be the COV for a 1.5 V cell when used in a flashlight. Some radio applications
can utilize the cell down to 0.75 V or lower, while other electronic devices may tolerate a drop
to only 1.2 V. Obviously, the lower the end voltage, the greater the amount of energy that can
be delivered by the battery. The lower voltage will impact certain applications, like flashlights,
resulting in a dimmer light and lower volume and/or range for radios. Devices that can operate
only within a narrow voltage range would do better with a battery system noted for a flat dis-
charge curve. Although a closed-circuit voltage that steadily decreases may present a disadvantage
in some applications, it is advantageous where sufficient warning of the end of battery life is
required, as in a flashlight.