Page 251 - Lindens Handbook of Batteries
P. 251
MAGNeSiUM AND ALUMiNUM bATTerieS 10.3
model involves, successively, metal dissolution at the metal-film interface, film dilatation, and film
breakdown. This wasteful reaction is a problem, not only because of the need to vent the hydrogen
from the battery and to prevent it from accumulating, but also because it uses water that is critical to
the battery operation, produces heat, and reduces the efficiency of the anode.
The efficiency of the magnesium anode is about 60 to 70% during a typical continuous discharge
and is influenced by such factors as the composition of the magnesium alloy, battery components,
discharge rate, and temperature. On low drains and intermittent service, the anode efficiency can
drop to 40 to 50% or less. The anode efficiency also is reduced with decreasing temperature.
Considerable heat is generated during the discharge of a magnesium battery, particularly at high
discharge rates, due to the exothermic corrosion reaction (about 82 kcal per gram-mole of magne-
sium) and the losses resulting from the difference between the theoretical and operating voltage.
proper battery design must allow for the dissipation of this heat to prevent overheating and shortened
life. On the other hand, this heat can be used to advantage at low ambient temperatures to maintain
the battery at higher and more efficient operating temperatures.
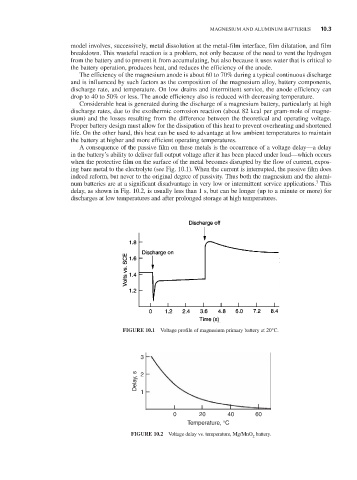
A consequence of the passive film on these metals is the occurrence of a voltage delay—a delay
in the battery’s ability to deliver full output voltage after it has been placed under load—which occurs
when the protective film on the surface of the metal becomes disrupted by the flow of current, expos-
ing bare metal to the electrolyte (see Fig. 10.1). When the current is interrupted, the passive film does
indeed reform, but never to the original degree of passivity. Thus both the magnesium and the alumi-
3
num batteries are at a significant disadvantage in very low or intermittent service applications. This
delay, as shown in Fig. 10.2, is usually less than 1 s, but can be longer (up to a minute or more) for
discharges at low temperatures and after prolonged storage at high temperatures.
FIGURE 10.1 Voltage profile of magnesium primary battery at 20°C.
3
Delay, s 2
1
0 20 40 60
Temperature, °C
FIGURE 10.2 Voltage delay vs. temperature, Mg/MnO battery.
2