Page 271 - Lindens Handbook of Batteries
P. 271
ALKALINE-MANGANESE DIOXIDE BATTERIES 11.9
in the type of carbon added to the alkaline cell cathode have occurred. Natural graphites, synthetic
graphites, acetylene black, and, most recently, expanded graphites have been used to improve the
cathode conductivity. In all cases, this conductor must be pure so as not to add any more impurities
to the cell. The expanded graphite allows less carbon to be used as its synthesis expands the graphite
7
planes, while maintaining its conductivity within the carbon planes. This graphite has a higher liq-
uid absorption value, and the particle size can be optimized for the required cathode formulation.
Other Components. KOH and water are used to form the cathode electrolyte. They are added dur-
ing the mixing of the cathode ingredients to form a moist paste. This makes the cathode mix easier
to handle and mold. Depending on the battery manufacturer, other ingredients, such as binders and
additives, are used to produce a dense and stable cathode with a good electronic and ionic conductiv-
ity. The battery must also perform efficiently under a variety of discharge conditions, including low
and high continuous and intermittent discharge over a wide range of temperatures.
11.3.2 Anode Components
The anode is composed of a mixture of ingredients that allow for good cell performance and provide
for easy manufacturing. The typical composition of an alkaline anode is listed in Table 11.6.
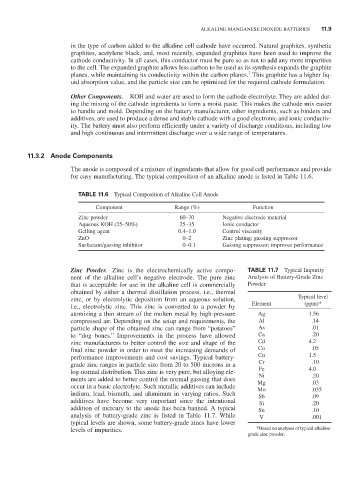
TABLE 11.6 Typical Composition of Alkaline Cell Anode
Component Range (%) Function
Zinc powder 60–70 Negative electrode material
Aqueous KOH (25–50%) 25–35 Ionic conductor
Gelling agent 0.4–1.0 Control viscosity
ZnO 0–2 Zinc plating; gassing suppressor
Surfactant/gassing inhibitor 0–0.1 Gassing suppressor; improves performance
Zinc Powder. Zinc is the electrochemically active compo- TABLE 11.7 Typical Impurity
nent of the alkaline cell’s negative electrode. The pure zinc Analysis of Battery-Grade Zinc
that is acceptable for use in the alkaline cell is commercially powder
obtained by either a thermal distillation process, i.e., thermal
zinc, or by electrolytic deposition from an aqueous solution, Typical level
i.e., electrolytic zinc. This zinc is converted to a powder by Element (ppm)*
atomizing a thin stream of the molten metal by high-pressure Ag 1.56
compressed air. Depending on the setup and requirements, the Al .14
particle shape of the obtained zinc can range from “potatoes” As .01
to “dog bones.” Improvements in the process have allowed Ca .20
zinc manufacturers to better control the size and shape of the Cd 4.2
final zinc powder in order to meet the increasing demands of Co .05
performance improvements and cost savings. Typical battery- Cu 1.5
grade zinc ranges in particle size from 20 to 500 microns in a Cr .10
log-normal distribution. This zinc is very pure, but alloying ele- Fe 4.0
ments are added to better control the normal gassing that does Ni .20
occur in a basic electrolyte. Such metallic additives can include Mg .03
Mo
.035
indium, lead, bismuth, and aluminum in varying ratios. Such Sb .09
additives have become very important since the intentional Si .20
addition of mercury to the anode has been banned. A typical Sn .10
analysis of battery-grade zinc is listed in Table 11.7. While V .001
typical levels are shown, some battery-grade zincs have lower
levels of impurities. *Based on analyses of typical alkaline-
grade zinc powder.