Page 285 - Lindens Handbook of Batteries
P. 285
MERCURIC OXIDE BATTERIES 12.5
With the exception of the anode contact (where slight modification of the top/anode interface
was necessary), materials for the cadmium/mercuric oxide cell were generally the same as for the
zinc/mercuric oxide cell. However, because of the wide range of storage and operating conditions
of most applications, cellulose and its derivatives were not used, and low-melting-point polymers
were also avoided. Nickel was usually used on the anode side of the cell and also, conveniently, at
the cathode.
12.4 CONSTRuCTION
The mercuric oxide batteries were manufactured in three basic structures—button, flat, and cylindri-
cal configurations. There were several design variations within each configuration.
12.4.1 Button Configuration
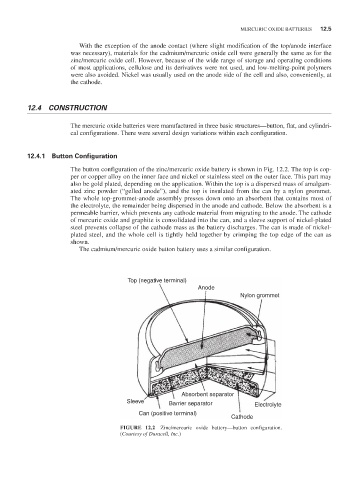
The button configuration of the zinc/mercuric oxide battery is shown in Fig. 12.2. The top is cop-
per or copper alloy on the inner face and nickel or stainless steel on the outer face. This part may
also be gold plated, depending on the application. Within the top is a dispersed mass of amalgam-
ated zinc powder (“gelled anode”), and the top is insulated from the can by a nylon grommet.
The whole top-grommet-anode assembly presses down onto an absorbent that contains most of
the electrolyte, the remainder being dispersed in the anode and cathode. Below the absorbent is a
permeable barrier, which prevents any cathode material from migrating to the anode. The cathode
of mercuric oxide and graphite is consolidated into the can, and a sleeve support of nickel-plated
steel prevents collapse of the cathode mass as the battery discharges. The can is made of nickel-
plated steel, and the whole cell is tightly held together by crimping the top edge of the can as
shown.
The cadmium/mercuric oxide button battery uses a similar configuration.
Top (negative terminal)
Anode
Nylon grommet
Absorbent separator
Sleeve Barrier separator Electrolyte
Can (positive terminal)
Cathode
FIGURE 12.2 Zinc/mercuric oxide battery—button configuration.
(Courtesy of Duracell, Inc.)