Page 283 - Lindens Handbook of Batteries
P. 283
MERCURIC OXIDE BATTERIES 12.3
12.3 CELL COmPONENTS
12.3.1 Electrolyte
Two types of alkaline electrolyte were used in the zinc/mercuric oxide cell, one based on potassium
hydroxide and one on sodium hydroxide. Both of these bases are very soluble in water and highly
concentrated solutions were used; zinc oxide was also dissolved in varying amounts in the solution
to suppress hydrogen generation.
Potassium hydroxide electrolytes generally contain between 30 and 45% w/w KOH and up to
7% w/w zinc oxide. They were more widely used than the sodium hydroxide electrolytes because
of their greater operating temperature range and ability to support heavier current drains. For low
temperature operation, both the potassium hydroxide and the zinc oxide contents were reduced,
and this introduced some instability at higher temperatures with respect to hydrogen generation in
the cell.
Sodium hydroxide electrolytes were pre- 90
pared in similar concentration ranges and were
used in cells where low temperature opera- 70
tions or high current drains were not required.
These electrolytes were suitable for long-term
discharge applications because of the reduced 50
tendency of the electrolyte to seep out of the
cell seal after long periods of storage. 30
Generally only potassium-based alkaline
electrolytes were used in the cadmium/mer- Temperature, °C 10 Liquid
curic oxide cell. As cadmium is practically
insoluble in all concentrations of aqueous –10
potassium hydroxide solutions, the electro-
lyte could be optimized for low-temperature –30
operation.
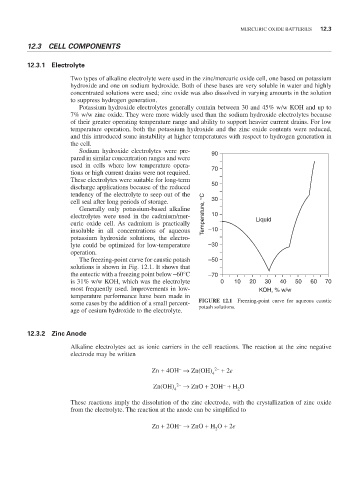
The freezing-point curve for caustic potash –50
solutions is shown in Fig. 12.1. It shows that
the eutectic with a freezing point below -60°C –70
is 31% w/w KOH, which was the electrolyte 0 10 20 30 40 50 60 70
most frequently used. Improvements in low- KOH, % w/w
temperature performance have been made in
some cases by the addition of a small percent- FIGURE 12.1 Freezing-point curve for aqueous caustic
potash solutions.
age of cesium hydroxide to the electrolyte.
12.3.2 Zinc Anode
Alkaline electrolytes act as ionic carriers in the cell reactions. The reaction at the zinc negative
electrode may be written
–
Zn + 4OH → Zn(OH) 4 2- + 2e
-
Zn(OH) 2- → ZnO + 2OH + H O
4 2
These reactions imply the dissolution of the zinc electrode, with the crystallization of zinc oxide
from the electrolyte. The reaction at the anode can be simplified to
Zn + 2OH → ZnO + H O + 2e
–
2