Page 307 - Lindens Handbook of Batteries
P. 307
BUTTOn CELL BATTErIES: SILVEr OxIDE–ZInC AnD ZInC-AIr SYSTEmS 13.13
8
1000 Hz
Impedance, ohms 4 100 Hz
6
2
20 40 60 80 100
Depth of discharge, %
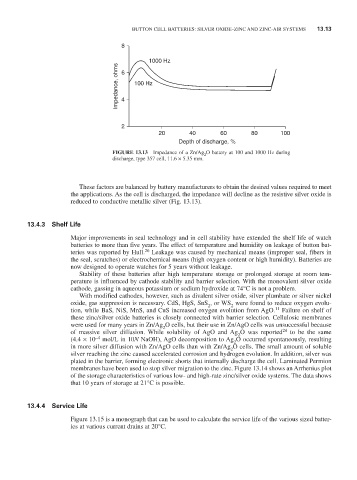
FiGURE 13.13 Impedance of a Zn/Ag O battery at 100 and 1000 Hz during
2
discharge, type 357 cell, 11.6 × 5.35 mm.
These factors are balanced by battery manufacturers to obtain the desired values required to meet
the applications. As the cell is discharged, the impedance will decline as the resistive silver oxide is
reduced to conductive metallic silver (Fig. 13.13).
13.4.3 shelf life
major improvements in seal technology and in cell stability have extended the shelf life of watch
batteries to more than five years. The effect of temperature and humidity on leakage of button bat-
teries was reported by Hull. Leakage was caused by mechanical means (improper seal, fibers in
26
the seal, scratches) or electrochemical means (high oxygen content or high humidity). Batteries are
now designed to operate watches for 5 years without leakage.
Stability of these batteries after high temperature storage or prolonged storage at room tem-
perature is influenced by cathode stability and barrier selection. With the monovalent silver oxide
cathode, gassing in aqueous potassium or sodium hydroxide at 74°C is not a problem.
With modified cathodes, however, such as divalent silver oxide, silver plumbate or silver nickel
oxide, gas suppression is necessary. CdS, HgS, SnS , or WS were found to reduce oxygen evolu-
2
2
11
tion, while BaS, niS, mnS, and CuS increased oxygen evolution from AgO. Failure on shelf of
these zinc/silver oxide batteries is closely connected with barrier selection. Cellulosic membranes
were used for many years in Zn/Ag O cells, but their use in Zn/AgO cells was unsuccessful because
2
of massive silver diffusion. While solubility of AgO and Ag O was reported to be the same
24
2
(4.4 × 10 mol/L in 10N naOH), AgO decomposition to Ag O occurred spontaneously, resulting
-4
2
in more silver diffusion with Zn/AgO cells than with Zn/Ag O cells. The small amount of soluble
2
silver reaching the zinc caused accelerated corrosion and hydrogen evolution. In addition, silver was
plated in the barrier, forming electronic shorts that internally discharge the cell. Laminated Permion
membranes have been used to stop silver migration to the zinc. Figure 13.14 shows an Arrhenius plot
of the storage characteristics of various low- and high-rate zinc/silver oxide systems. The data shows
that 10 years of storage at 21°C is possible.
13.4.4 service life
Figure 13.15 is a monograph that can be used to calculate the service life of the various sized batter-
ies at various current drains at 20°C.