Page 312 - Lindens Handbook of Batteries
P. 312
13.18 PrImArY BATTErIES
solubility of the additives placed in the cathode, and the degree to which they will promote parasitic
reactions in the anode if they dissolve into the electrolyte over time.
13.8 CONSTRUCTION
Button and coin cells are the most prevalent form for zinc/air batteries to take. Development of other
formats, such as cylindrical and prismatic forms, has been somewhat limited due to economics, but
also by the frustrations of air management and the poor performance of zinc/air when use is intermit-
tent. moisture sensitivity in large cells becomes a greater problem when rate capability demands a
largely open cathode to air interface. Unless the cathode can be isolated from the environment during
the idle periods, the cells will suffer the adverse effects of water gain or loss, as well as the degrada-
tion caused by the accumulation of carbonates at the air-electrolyte interface.
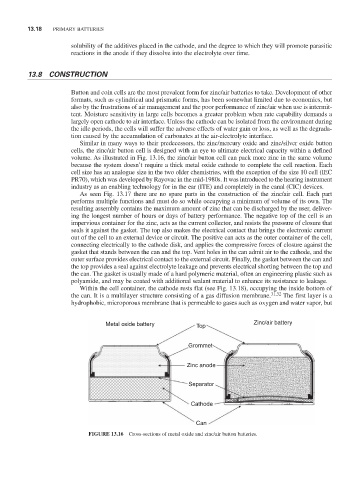
Similar in many ways to their predecessors, the zinc/mercury oxide and zinc/silver oxide button
cells, the zinc/air button cell is designed with an eye to ultimate electrical capacity within a defined
volume. As illustrated in Fig. 13.16, the zinc/air button cell can pack more zinc in the same volume
because the system doesn’t require a thick metal oxide cathode to complete the cell reaction. Each
cell size has an analogue size in the two older chemistries, with the exception of the size 10 cell (IEC
Pr70), which was developed by rayovac in the mid-1980s. It was introduced to the hearing instrument
industry as an enabling technology for in the ear (ITE) and completely in the canal (CIC) devices.
As seen Fig. 13.17 there are no spare parts in the construction of the zinc/air cell. Each part
performs multiple functions and must do so while occupying a minimum of volume of its own. The
resulting assembly contains the maximum amount of zinc that can be discharged by the user, deliver-
ing the longest number of hours or days of battery performance. The negative top of the cell is an
impervious container for the zinc, acts as the current collector, and resists the pressure of closure that
seals it against the gasket. The top also makes the electrical contact that brings the electronic current
out of the cell to an external device or circuit. The positive can acts as the outer container of the cell,
connecting electrically to the cathode disk, and applies the compressive forces of closure against the
gasket that stands between the can and the top. Vent holes in the can admit air to the cathode, and the
outer surface provides electrical contact to the external circuit. Finally, the gasket between the can and
the top provides a seal against electrolyte leakage and prevents electrical shorting between the top and
the can. The gasket is usually made of a hard polymeric material, often an engineering plastic such as
polyamide, and may be coated with additional sealant material to enhance its resistance to leakage.
Within the cell container, the cathode rests flat (see Fig. 13.18), occupying the inside bottom of
the can. It is a multilayer structure consisting of a gas diffusion membrane. 31,32 The first layer is a
hydrophobic, microporous membrane that is permeable to gases such as oxygen and water vapor, but
Metal oxide battery Top Zinc/air battery
Grommet
Zinc anode
Separator
Cathode
Can
FiGURE 13.16 Cross-sections of metal oxide and zinc/air button batteries.