Page 339 - Lindens Handbook of Batteries
P. 339
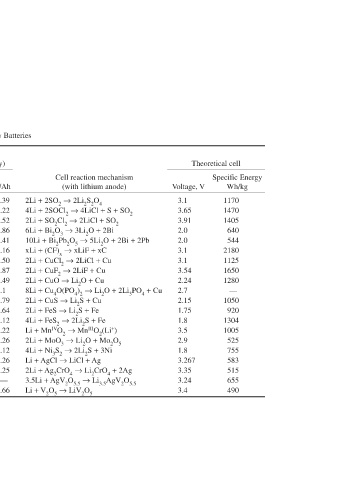
Theoretical cell Specific energy Wh/kg 1170 1470 1405 640 544 2180 1125 1650 1280 — 1050 920 1304 1005 525 755 583 515 655 490
Voltage, V 3.1 3.65 3.91 2.0 2.0 3.1 3.1 3.54 2.24 2.7 2.15 1.75 1.8 3.5 2.9 1.8 3.267 3.35 3.24 3.4
Cell reaction mechanism (with lithium anode) 2Li + 2SO 2 → 2Li 2 S 2 O 4 4Li + 2SOCl 2 → 4LiCl + S + SO 2 2Li + SO 2 Cl 2 → 2LiCl + SO 2 6Li + Bi 2 O 3 → 3Li 2 O + 2Bi 10Li + Bi 2 Pb 2 O 5 → 5Li 2 O + 2Bi + 2Pb xLi + (CF) x → xLiF + xC 2Li + CuCl 2 → 2LiCl + Cu 2Li + CuF 2 → 2LiF + Cu 2Li + CuO → Li 2 O + Cu 8Li + Cu 4 O(PO 4 ) 2 → Li 2 O + 2Li 3 PO 4 + Cu 2Li + CuS → Li 2 S + Cu 2Li + FeS → Li 2 S + Fe 4Li + FeS 2 → 2Li 2 S + Fe Li + Mn iV O 2 → Mn i
Cathode Materials Currently or Previously Used in Lithium Primary Batteries
Theoretical faradic capacity (cathode only) g/Ah Ah/cm 3 2.39 — 2.22 — 2.52 — 2.86 2.97 2.41 2.64 1.16 2.32 2.50 1.22 1.87 1.52 1.49 4.26 2.1 — 1.79 2.57 1.64 2.95 1.12 4.35 3.22 1.54 5.26 0.84 2.12 — 5.26 1.04 6.25 0.90 — — 6.66 0.53
Density, Ah/g g/cm 3 0.419 1.37 0.450 1.63 0.397 1.66 0.35 8.5 0.29 9.0 0.86 2.7 0.40 3.1 0.53 2.9 0.67 6.4 0.468 — 0.56 4.6 0.61 4.8 0.89 4.9 0.31 5.0 0.19 4.5 0.47 — 0.19 5.6 0.16 5.6 0.282 — 0.15 3.6
Valence change 1 2 2 6 10 1 2 2 2 8 2 2 4 1 1 4 1 2 3.5 1 *Multiple-step discharge; see ref. 11 (experimental values to +1.5 V cutoff).
Molecular weight 64 119 135 466 912 31 134.5 101.6 79.6 458.3 95.6 87.9 119.9 86.9 143 240 143.3 331.8 297.7 181.9
TABLE 14.4 Cathode material SO 2 SOCl 2 SO 2Cl 2 Bi 2 O 3 Bi 2 Pb 2 O 5 (CF) x CuCl 2 CuF 2 CuO Cu 4 O(PO 4 ) 2 CuS FeS FeS 2 MnO 2 MoO 3 Ni 3 S 2 AgCl Ag 2 CrO 4 AgV 2 O 5.5 * V 2 O 5
14.6