Page 48 - Lindens Handbook of Batteries
P. 48
ELECTROCHEMICAL PRINCIPLES AND REACTIONS 2.5
reaction. As in any reaction, the overall rate of the electrochemical process is determined by the rate
of the slowest step in the whole sequence of reactions.
The thermodynamic treatment of electrochemical processes presented in Sec. 2.2 describes the
equilibrium condition of a system but does not present information on nonequilibrium conditions
such as current flow resulting from electrode polarization (overvoltage) imposed to effect electro-
chemical reactions. Experimental determination of the current-voltage characteristics of many elec-
trochemical systems has shown that there is an exponential relation between current and applied voltage.
The generalized expression describing this relationship is called the Tafel equation,
i
η= ±ablog (2.7)
where η = overvoltage, i = current, and a and b are constants. Typically, the constant b is referred to
as the Tafel slope. The Tafel relationship holds for a large number of electrochemical systems over
a wide range of overpotentials. At low values of overvoltage, however, the relationship breaks down
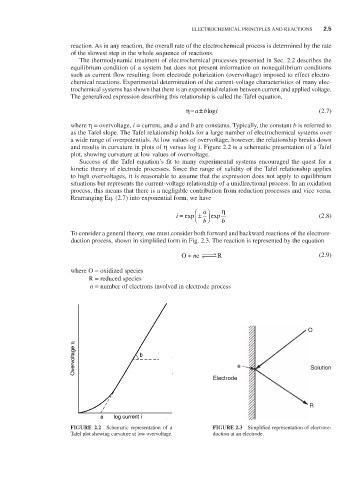
and results in curvature in plots of η versus log i. Figure 2.2 is a schematic presentation of a Tafel
plot, showing curvature at low values of overvoltage.
Success of the Tafel equation’s fit to many experimental systems encouraged the quest for a
kinetic theory of electrode processes. Since the range of validity of the Tafel relationship applies
to high overvoltages, it is reasonable to assume that the expression does not apply to equilibrium
situations but represents the current-voltage relationship of a unidirectional process. In an oxidation
process, this means that there is a negligible contribution from reduction processes and vice versa.
Rearranging Eq. (2.7) into exponential form, we have
i = ± exp a exp η (2.8)
b b
To consider a general theory, one must consider both forward and backward reactions of the electrore-
duction process, shown in simplified form in Fig. 2.3. The reaction is represented by the equation
O+ e R (2.9)
n
where O = oxidized species
R = reduced species
n = number of electrons involved in electrode process
O
e Solution
Electrode
R
FIGURE 2.2 Schematic representation of a FIGURE 2.3 Simplified representation of electrore-
Tafel plot showing curvature at low overvoltage. duction at an electrode.