Page 45 - Lindens Handbook of Batteries
P. 45
2.2 PRINCIPLES OF OPERATION
When connected to an external load R, the cell voltage E can be expressed as
E = E - 0 ct a +[(η c a - (η ctc +[(η cc - (η iR = iR (2.1)
)
)]
)
)]
i
where E = electromotive force or open-circuit voltage of cell
o
( η ct a η ctc
) = activation polarization or charge-transfer overvoltage at anode and cathode
) ,(
) = concentration polarization at anode and cathode
) ,(
( η ca η cc
i = operating current of cell on load
R = internal resistance of cell
i
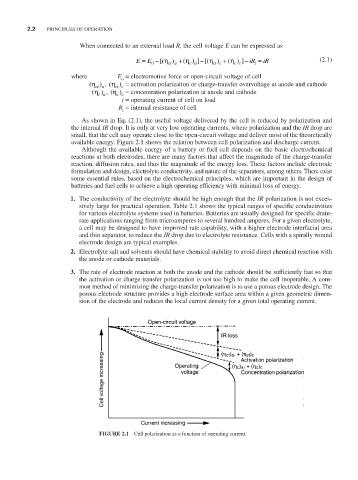
As shown in Eq. (2.1), the useful voltage delivered by the cell is reduced by polarization and
the internal IR drop. It is only at very low operating currents, where polarization and the IR drop are
small, that the cell may operate close to the open-circuit voltage and deliver most of the theoretically
available energy. Figure 2.1 shows the relation between cell polarization and discharge current.
Although the available energy of a battery or fuel cell depends on the basic electrochem ical
reactions at both electrodes, there are many factors that affect the magnitude of the charge-transfer
reaction, diffusion rates, and thus the magnitude of the energy loss. These factors include electrode
formulation and design, electrolyte conductivity, and nature of the separators, among others. There exist
some essential rules, based on the electrochemical principles, which are important in the design of
batteries and fuel cells to achieve a high operating efficiency with minimal loss of energy.
1. The conductivity of the electrolyte should be high enough that the IR polarization is not exces-
sively large for practical operation. Table 2.1 shows the typical ranges of specific conductivities
for various electrolyte systems used in batteries. Batteries are usually designed for specific drain-
rate applications ranging from microamperes to several hundred amperes. For a given electrolyte,
a cell may be designed to have improved rate capability, with a higher electrode interfacial area
and thin separator, to reduce the IR drop due to electrolyte resistance. Cells with a spirally wound
electrode design are typical examples.
2. Electrolyte salt and solvents should have chemical stability to avoid direct chemical reaction with
the anode or cathode materials.
3. The rate of electrode reaction at both the anode and the cathode should be sufficiently fast so that
the activation or charge-transfer polarization is not too high to make the cell inoperable. A com-
mon method of minimizing the charge-transfer polarization is to use a porous electrode design. The
porous electrode structure provides a high electrode surface area within a given geometric dimen-
sion of the electrode and reduces the local current density for a given total operating current.
FIGURE 2.1 Cell polarization as a function of operating current.