Page 41 - Lindens Handbook of Batteries
P. 41
1.16 PRINCIPLES OF OPERATION
1000
Lithium (cylindrical) Zinc/air
Specific energy, Wh/kg Alkaline MnO 2 Zn/Ag 2 O
500
Lithium (coin)
100
Zn/HgO
Carbon-zinc
50
100 500 1000 5000
Energy density, Wh/L
(a)
300
Specific energy (Wh/kg) 200 Ni-Cd Li-ion
100
0
0 100 200 NiMH 300 400 500 600
Energy density (Wh/L)
(b)
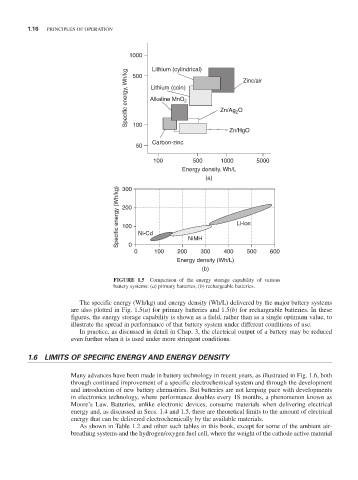
FIGURE 1.5 Comparison of the energy storage capability of various
battery systems: (a) primary batteries, (b) rechargeable batteries.
The specific energy (Wh/kg) and energy density (Wh/L) delivered by the major battery systems
are also plotted in Fig. 1.5(a) for primary batteries and 1.5(b) for rechargeable batteries. In these
figures, the energy storage capability is shown as a field, rather than as a single optimum value, to
illustrate the spread in performance of that battery system under different conditions of use.
In practice, as discussed in detail in Chap. 3, the electrical output of a battery may be reduced
even further when it is used under more stringent conditions.
1.6 LIMITS OF SPECIFIC ENERGY AND ENERGY DENSITY
Many advances have been made in battery technology in recent years, as illustrated in Fig. 1.6, both
through continued improvement of a specific electrochemical system and through the development
and introduction of new battery chemistries. But batteries are not keeping pace with developments
in electronics technology, where performance doubles every 18 months, a phenomenon known as
Moore’s Law. Batteries, unlike electronic devices, consume materials when delivering electrical
energy and, as discussed in Secs. 1.4 and 1.5, there are theoretical limits to the amount of electrical
energy that can be delivered electrochemically by the available materials.
As shown in Table 1.2 and other such tables in this book, except for some of the ambient air-
breathing systems and the hydrogen/oxygen fuel cell, where the weight of the cathode active material