Page 37 - Lindens Handbook of Batteries
P. 37
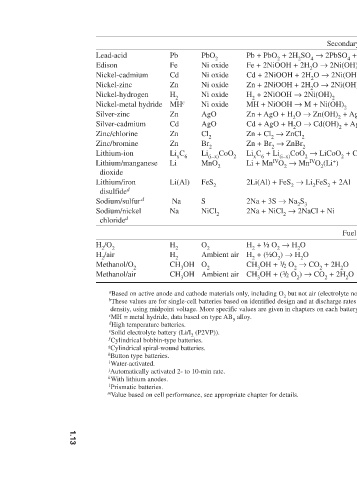
Cd + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Cd(OH) 2
Zn + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Zn(OH) 2
Fe + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Fe(OH) 2
Secondary batteries 70 l 35 2.0 252 120 8.32 2.1 55 l 30 1.2 314 224 4.46 1.4 135 g 40 1.2 244 181 5.52 1.35 185 90 1.6 372 215 4.64 1.73 60 55 1.2 434 289 3.46 1.5 235 g 100 1.2 240 178 5.63 1.35 180 l 105 1.5 524 283 3.53 1.85 120 l 70 1.1 318 227 4.41 1.4 — — — 835 394 2.54 2.12 60 70 1.6 572 309 4.17 1.85 570 g 200 3.8 448 109 9.14 4.1 265
Pb + PbO 2 + 2H 2 SO 4 → 2PbSO 4 + 2H 2 O
H 2 + 2NiOOH → 2Ni(OH) 2 MH + NiOOH → M + Ni(OH) 2 Zn + AgO + H 2 O → Zn(OH) 2 + Ag Cd + AgO + H 2 O → Cd(OH) 2 + Ag Zn + Cl 2 → ZnCl 2 Zn + Br 2 → ZnBr 2 Li x C 6 + Li (i–x) CoO 2 → LiCoO 2 + C 6 Li + Mn IV O 2 → Mn IV O 2 (Li + ) 2Li(Al) + FeS 2 → Li 2 FeS 2 + 2Al 2Na + 3S → Na 2 S 3 2Na + NiCl 2 → 2NaCl + Ni H 2 + ½ O 2 → H 2 O H 2 + (½O 2 ) → H 2 O CH 3 OH + 3 / 2 O 2 → CO 2 + 2H 2 O CH 3 OH + ( 3 / 2 O 2 ) → CO 2 + 2H 2 O b These values are for single-cell batterie
PbO 2 Ni oxide Ni oxide Ni oxide Ni oxide Ni oxide AgO AgO Cl 2 Br 2 Li (i–x) CoO 2 MnO 2 FeS 2 S NiCl 2 O 2 Ambient air O 2 Ambient air a Based on active anode and cathode materials only, including O 2 but not air (electrolyte not included). density, using midpoint voltage. More specific values are given in chapters on each battery system. m Value based on cell performance, see appropriate chapter for details.
Pb Fe Cd Zn H 2 MH c Zn Cd Zn Zn Li x C 6 Li Li(Al) Na Na H 2 H 2 CH 3 OH CH 3 OH c MH = metal hydride, data based on type AB 5 alloy. d High temperature batteries. e Solid electrolyte battery (Li/I 2 (P2VP)). f Cylindrical bobbin-type batteries. g Cylindrical spiral-wound batteries. j Automatically activated 2- to 10-min rate.
Lead-acid Edison Nickel-cadmium Nickel-zinc Nickel-hydrogen Nickel-metal hydride Silver-zinc Silver-cadmium Zinc/chlorine Zinc/bromine Lithium-ion Lithium/manganese dioxide Lithium/iron disulfide d Sodium/sulfur d Sodium/nickel chloride d H 2 /O 2 H 2 /air Methanol/O 2 Methanol/air h Button type batteries. i Water-activated. k With lithium anodes. l Prismatic batteries.
1.13