Page 35 - Lindens Handbook of Batteries
P. 35
Energy
Practical battery b
Specific
Nominal
Specific
Theoretical values a
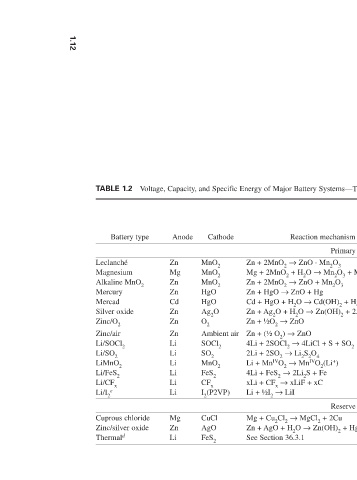
Voltage, Capacity, and Specific Energy of Major Battery Systems—Theoretical and Practical Values
Reaction mechanism density energy voltage energy Wh/L Wh/kg V Wh/kg Ah/kg g /Ah V Primary batteries 165 f 85 f 1.5 358 224 4.46 1.6 Zn + 2MnO 2 → ZnO · Mn 2 O 3 195 f 100 f 1.7 759 271 3.69 2.8 Mg + 2MnO 2 + H 2 O → Mn 2 O 3 + Mg(OH) 2 461 f 154 f 1.5 358 224 4.46 1.5 Zn + 2MnO 2 → ZnO + Mn 2 O 3 470 h 100 h 1.35 255 190 5.27 1.34 Zn + HgO → ZnO + Hg 230 h 55 h 0.9 148 163
Cathode MnO 2 MnO 2 MnO 2 HgO HgO Ag 2 O O 2 Ambient air SOCl 2 SO 2 MnO 2 FeS 2 CF x Li + ½I 2 → LiI I 2 (P2VP) CuCl AgO FeS 2
Anode Zn Mg Zn Zn Cd Zn Zn Zn Li Li Li Li Li Li Mg Zn Li
TABLE 1.2 Battery type Leclanché Magnesium Alkaline MnO 2 Mercury Mercad Silver oxide Zinc/O 2 Zinc/air Li/SOCl 2 Li/SO 2 LiMnO 2 Li/FeS 2 Li/CF x Li/I 2 e Cuprous chloride Zinc/silver oxide Thermal d
1.12