Page 33 - Lindens Handbook of Batteries
P. 33
1.10 PRINCIPLES OF OPERATION
Anode (oxidation potential) + cathode (reduction potential) = standard cell potential
For example, in the reaction Zn + Cl → ZnCl , the standard cell potential is:
2
2
Zn → Zn + 2 + 2e −−0 76 V)
.
(
.
Cl → Cl −2 − 2e 136 V
2
.
E °= 212 V
The cell voltage is also dependent on other factors, including concentration and temperature, as
expressed by the Nernst equation (covered in detail in Chap. 2).
1.4.3 Theoretical Capacity (Coulombic)
The theoretical capacity of a cell is determined by the amount of active materials in the cell. It is
expressed as the total quantity of electricity involved in the electrochemical reaction and is defined in
terms of coulombs or ampere-hours. The “ampere-hour capacity” of a battery is directly associated with
the quantity of electricity obtained from the active materials. Theoretically, 1 gram-equivalent weight of
material will deliver 96,487 C or 26.8 Ah. (A gram-equivalent weight is the atomic or molecular weight
of the active material in grams divided by the number of electrons involved in the reaction.)
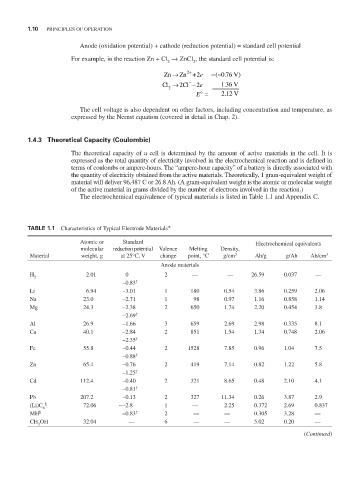
The electrochemical equivalence of typical materials is listed in Table 1.1 and Appendix C.
TABLE 1.1 Characteristics of Typical Electrode Materials*
Atomic or Standard Electrochemical equivalents
molecular reduction potential Valence Melting Density,
Material weight, g at 25°C, V change point, °C g/cm 3 Ah/g g/Ah Ah/cm 3
Anode materials
H 2.01 0 2 — — 26.59 0.037 —
2
–0.83 †
Li 6.94 –3.01 1 180 0.54 3.86 0.259 2.06
Na 23.0 –2.71 1 98 0.97 1.16 0.858 1.14
Mg 24.3 –2.38 2 650 1.74 2.20 0.454 3.8
–2.69 †
Al 26.9 –1.66 3 659 2.69 2.98 0.335 8.1
Ca 40.1 –2.84 2 851 1.54 1.34 0.748 2.06
–2.35 †
Fe 55.8 –0.44 2 1528 7.85 0.96 1.04 7.5
–0.88 †
Zn 65.4 –0.76 2 419 7.14 0.82 1.22 5.8
–1.25 †
Cd 112.4 –0.40 2 321 8.65 0.48 2.10 4.1
–0.81 †
Pb 207.2 –0.13 2 327 11.34 0.26 3.87 2.9
(Li)C § 72.06 ∼ –2.8 1 — 2.25 0.372 2.69 0.837
6
MH ¶ –0.83 † 2 — — 0.305 3.28 —
CH OH 32.04 — 6 — — 5.02 0.20 —
3
(Continued)