Page 31 - Lindens Handbook of Batteries
P. 31
1.8 PRINCIPLES OF OPERATION
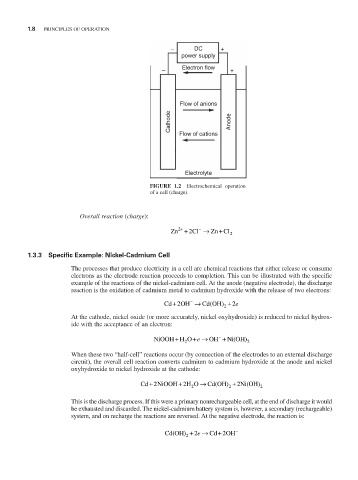
– DC +
power supply
– Electron flow +
Flow of anions
Cathode Anode
Flow of cations
Electrolyte
FIGURE 1.2 Electrochemical operation
of a cell (charge).
Overall reaction (charge):
Zn + 2 + 2 Cl → − Zn Cl
+
2
1.3.3 Specific Example: Nickel-Cadmium Cell
The processes that produce electricity in a cell are chemical reactions that either release or consume
electrons as the electrode reaction proceeds to completion. This can be illustrated with the specific
example of the reactions of the nickel-cadmium cell. At the anode (negative electrode), the discharge
reaction is the oxidation of cadmium metal to cadmium hydroxide with the release of two electrons:
)
(
Cd+ OH → 2 − Cd OH + 2e
2
At the cathode, nickel oxide (or more accurately, nickel oxyhydroxide) is reduced to nickel hydrox-
ide with the acceptance of an electron:
+
NiOOH HO+ e → OH + − Ni(OH)
2 2
When these two “half-cell” reactions occur (by connection of the electrodes to an external discharge
circuit), the overall cell reaction converts cadmium to cadmium hydroxide at the anode and nickel
oxyhydroxide to nickel hydroxide at the cathode:
Cd+ NiOOH 2H O → + 2 Cd(OH) + 2Ni(OH)
2 2 2
This is the discharge process. If this were a primary nonrechargeable cell, at the end of discharge it would
be exhausted and discarded. The nickel-cadmium battery system is, however, a secondary (rechargeable)
system, and on recharge the reactions are reversed. At the negative electrode, the reaction is:
Cd(OH) + → 2e Cd+ 2 OH −
2