Page 36 - Lindens Handbook of Batteries
P. 36
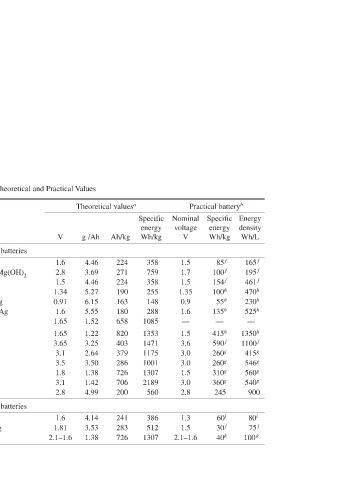
Energy density Wh/L 165 f 195 f 461 f 470 h 230 h 525 h — 1350 h 1100 f 415 g 546 g 560 g 540 g 900 80 i 75 j 100 k
Practical battery b Specific energy Wh/kg 85 f 100 f 154 f 100 h 55 h 135 h — 415 h 590 f 260 g 260 g 310 g 360 g 245 60 i 30 j 40 k
Nominal voltage V 1.5 1.7 1.5 1.35 0.9 1.6 — 1.5 3.6 3.0 3.0 1.5 3.0 2.8 1.3 1.5 2.1–1.6
Specific energy Wh/kg 358 759 358 255 148 288 1085 1353 1471 1175 1001 1307 2189 560 386 512 1307
Theoretical values a Ah/kg g /Ah 224 4.46 271 3.69 224 4.46 190 5.27 163 6.15 180 5.55 658 1.52 820 1.22 403 3.25 379 2.64 286 3.50 726 1.38 706 1.42 200 4.99 241 4.14 283 3.53 726 1.38
Voltage, Capacity, and Specific Energy of Major Battery Systems—Theoretical and Practical Values
V 1.6 2.8 1.5 1.34 0.91 1.6 1.65 1.65 3.65 3.1 3.5 1.8 3.1 2.8 1.6 1.81 2.1–1.6
Primary batteries Reserve batteries
Reaction mechanism Zn + 2MnO 2 → ZnO · Mn 2 O 3 Mg + 2MnO 2 + H 2 O → Mn 2 O 3 + Mg(OH) 2 Zn + 2MnO 2 → ZnO + Mn 2 O 3 Zn + HgO → ZnO + Hg Cd + HgO + H 2 O → Cd(OH) 2 + Hg Zn + Ag 2 O + H 2 O → Zn(OH) 2 + 2Ag Zn + ½O 2 → ZnO Zn + (½ O 2 ) → ZnO 4Li + 2SOCl 2 → 4LiCl + S + SO 2 2Li + 2SO 2 → Li 2 S 2 O 4 Li + Mn IV O 2 → Mn IV O 2 (Li + ) 4Li + FeS 2 → 2Li 2 S + Fe xLi + CF x → xLiF + xC Mg + Cu 2 Cl 2 → MgCl 2 + 2Cu Zn + AgO + H 2 O → Z
Cathode MnO 2 MnO 2 MnO 2 HgO HgO Ag 2 O O 2 Ambient air SOCl 2 SO 2 MnO 2 FeS 2 CF x Li + ½I 2 → LiI I 2 (P2VP) CuCl AgO FeS 2
Anode Zn Mg Zn Zn Cd Zn Zn Zn Li Li Li Li Li Li Mg Zn Li
TABLE 1.2 Battery type Leclanché Magnesium Alkaline MnO 2 Mercury Mercad Silver oxide Zinc/O 2 Zinc/air Li/SOCl 2 Li/SO 2 LiMnO 2 Li/FeS 2 Li/CF x Li/I 2 e Cuprous chloride Zinc/silver oxide Thermal d
1.12