Page 38 - Lindens Handbook of Batteries
P. 38
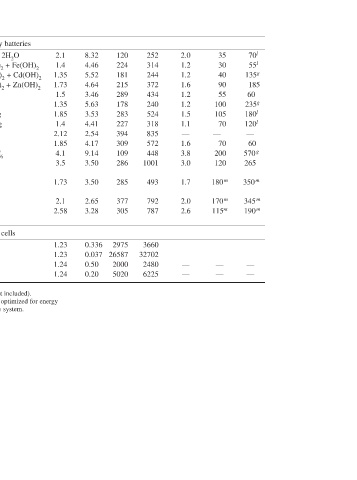
70 l 55 l 135 g 185 60 235 g 180 l 120 l — 60 570 g 265 350 m 345 m 190 m — —
35 30 40 90 55 100 105 70 70 200 120 180 m 170 m 115 m — —
—
2.0 1.2 1.2 1.6 1.2 1.2 1.5 1.1 — 1.6 3.8 3.0 1.7 2.0 2.6 — —
252 314 244 372 434 240 524 318 835 572 448 1001 493 792 787 3660 32702 2480 6225
120 224 181 215 289 178 283 227 394 309 109 286 285 377 305 2975 26587 2000 5020
8.32 4.46 5.52 4.64 3.46 5.63 3.53 4.41 2.54 4.17 9.14 3.50 3.50 2.65 3.28 0.336 0.037 0.50 0.20
2.1 1.4 1.35 1.73 1.5 1.35 1.85 1.4 2.12 1.85 4.1 3.5 1.73 2.1 2.58 1.23 1.23 1.24 1.24
Cd + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Cd(OH) 2
Zn + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Zn(OH) 2
Fe + 2NiOOH + 2H 2 O → 2Ni(OH) 2 + Fe(OH) 2
Secondary batteries Fuel cells
Pb + PbO 2 + 2H 2 SO 4 → 2PbSO 4 + 2H 2 O
H 2 + 2NiOOH → 2Ni(OH) 2 MH + NiOOH → M + Ni(OH) 2 Zn + AgO + H 2 O → Zn(OH) 2 + Ag Cd + AgO + H 2 O → Cd(OH) 2 + Ag Zn + Cl 2 → ZnCl 2 Zn + Br 2 → ZnBr 2 Li x C 6 + Li (i–x) CoO 2 → LiCoO 2 + C 6 Li + Mn IV O 2 → Mn IV O 2 (Li + ) 2Li(Al) + FeS 2 → Li 2 FeS 2 + 2Al 2Na + 3S → Na 2 S 3 2Na + NiCl 2 → 2NaCl + Ni H 2 + ½ O 2 → H 2 O H 2 + (½O 2 ) → H 2 O CH 3 OH + 3 / 2 O 2 → CO 2 + 2H 2 O CH 3 OH + ( 3 / 2 O 2 ) → CO 2 + 2H 2 O b These values are for single-cell batterie
PbO 2 Ni oxide Ni oxide Ni oxide Ni oxide Ni oxide AgO AgO Cl 2 Br 2 Li (i–x) CoO 2 MnO 2 FeS 2 S NiCl 2 O 2 Ambient air O 2 Ambient air a Based on active anode and cathode materials only, including O 2 but not air (electrolyte not included). density, using midpoint voltage. More specific values are given in chapters on each battery system. m Value based on cell performance, see appropriate chapter for details.
Pb Fe Cd Zn H 2 MH c Zn Cd Zn Zn Li x C 6 Li Li(Al) Na Na H 2 H 2 CH 3 OH CH 3 OH c MH = metal hydride, data based on type AB 5 alloy. d High temperature batteries. e Solid electrolyte battery (Li/I 2 (P2VP)). f Cylindrical bobbin-type batteries. g Cylindrical spiral-wound batteries. j Automatically activated 2- to 10-min rate.
Lead-acid Edison Nickel-cadmium Nickel-zinc Nickel-hydrogen Nickel-metal hydride Silver-zinc Silver-cadmium Zinc/chlorine Zinc/bromine Lithium-ion Lithium/manganese dioxide Lithium/iron disulfide d Sodium/sulfur d Sodium/nickel chloride d H 2/O 2 H 2/air Methanol/O 2 Methanol/air h Button type batteries. i Water-activated. k With lithium anodes. l Prismatic batteries.
1.13