Page 254 - Low Temperature Energy Systems with Applications of Renewable Energy
P. 254
240 Low-Temperature Energy Systems with Applications of Renewable Energy
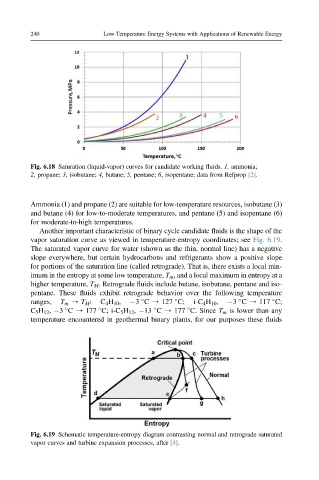
Fig. 6.18 Saturation (liquid-vapor) curves for candidate working fluids. 1, ammonia;
2, propane; 3, isobutane; 4, butane; 5, pentane; 6, isopentane; data from Refprop [2].
Ammonia (1) and propane (2) are suitable for low-temperature resources, isobutane (3)
and butane (4) for low-to-moderate temperatures, and pentane (5) and isopentane (6)
for moderate-to-high temperatures.
Another important characteristic of binary cycle candidate fluids is the shape of the
vapor saturation curve as viewed in temperature-entropy coordinates; see Fig. 6.19.
The saturated vapor curve for water (shown as the thin, normal line) has a negative
slope everywhere, but certain hydrocarbons and refrigerants show a positive slope
for portions of the saturation line (called retrograde). That is, there exists a local min-
imum in the entropy at some low temperature, T m , and a local maximum in entropy at a
higher temperature, T M . Retrograde fluids include butane, isobutane, pentane and iso-
pentane. These fluids exhibit retrograde behavior over the following temperature
ranges, T m / T M : C 4 H 10 , 3 C / 127 C; i-C 4 H 10 , 3 C / 117 C;
C 5 H 12 , 3 C / 177 C; i-C 5 H 12 , 13 C / 177 C. Since T m is lower than any
temperature encountered in geothermal binary plants, for our purposes these fluids
Fig. 6.19 Schematic temperature-entropy diagram contrasting normal and retrograde saturated
vapor curves and turbine expansion processes, after [4].