Page 50 - Macromolecular Crystallography
P. 50
HIGH-THROUGHPUT CLONING, EXPRESSION, AND PURIFICATION 39
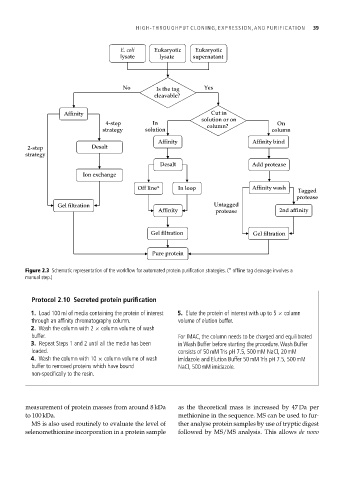
E. coli Eukaryotic Eukaryotic
lysate lysate supernatant
No Is the tag Yes
cleavable?
Affinity Cut in
solution or on
4-step In column? On
strategy solution column
Affinity Affinity bind
2-step Desalt
strategy
Desalt Add protease
Ion exchange
Off line* In loop Affinity wash
Tagged
protease
Gel filtration Untagged
Affinity protease 2nd affinity
Gel filtration Gel filtration
Pure protein
Figure 2.3 Schematic representation of the workflow for automated protein purification strategies. (* offline tag cleavage involves a
manual step.)
Protocol 2.10 Secreted protein purification
1. Load 100 ml of media containing the protein of interest 5. Elute the protein of interest with up to 5 × column
through an affinity chromatography column. volume of elution buffer.
2. Wash the column with 2 × column volume of wash
buffer. For IMAC, the column needs to be charged and equilibrated
3. Repeat Steps 1 and 2 until all the media has been in Wash Buffer before starting the procedure. Wash Buffer
loaded. consists of 50 mM Tris pH 7.5, 500 mM NaCl, 20 mM
4. Wash the column with 10 × column volume of wash imidazole and Elution Buffer 50 mM Tris pH 7.5, 500 mM
buffer to removed proteins which have bound NaCl, 500 mM imidazole.
non-specifically to the resin.
measurement of protein masses from around 8 kDa as the theoretical mass is increased by 47 Da per
to 100 kDa. methionine in the sequence. MS can be used to fur-
MS is also used routinely to evaluate the level of ther analyse protein samples by use of tryptic digest
selenomethionine incorporation in a protein sample followed by MS/MS analysis. This allows de novo