Page 59 - Macromolecular Crystallography
P. 59
48 MACROMOLECULAR CRYS TALLOGRAPHY
benefits of a microbatch experiment while gaining
Dispensing tip the inherent advantage of the self-screening process
of a diffusion trial (D’Arcy et al., 2003). This modifi-
cation is based on the following rationale: water can
evaporate through different oils at different rates.
Paraffin oil acts as a good sealant allowing only
a negligible amount of water evaporation through
the paraffin oil during the average time required
for a crystallization experiment. In contrast, water
Low density can diffuse freely through silicone oils. A mixture
oil
of paraffin and silicone oils permits partial diffu-
sion, depending on the ratio at which they are mixed
Initial position
of crystallization (D’Arcy et al., 1996).
drop
Crystallization It has been shown that for screening purposes it
plate is preferable to use silicone oil or a mixture of paraf-
Final position
of crystallization fin and silicone oils (D’Arcy et al., 1996, 2003). This
drop allows some evaporation of the drops, leading to a
higher number of ‘hits’ and faster formation of crys-
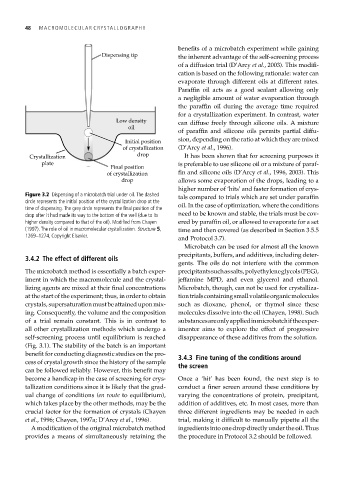
Figure 3.2 Dispensing of a microbatch trial under oil. The dashed tals compared to trials which are set under paraffin
circle represents the initial position of the crystallization drop at the
time of dispensing. The grey circle represents the final position of the oil. In the case of optimization, where the conditions
drop after it had made its way to the bottom of the well (due to its need to be known and stable, the trials must be cov-
higher density compared to that of the oil). Modified from Chayen ered by paraffin oil, or allowed to evaporate for a set
(1997). The role of oil in macromolecular crystallization. Structure 5, time and then covered (as described in Section 3.5.5
1269–1274, Copyright Elsevier.
and Protocol 3.7).
Microbatch can be used for almost all the known
precipitants, buffers, and additives, including deter-
3.4.2 The effect of different oils
gents. The oils do not interfere with the common
The microbatch method is essentially a batch exper- precipitantssuchassalts,polyethyleneglycols(PEG),
iment in which the macromolecule and the crystal- jeffamine MPD, and even glycerol and ethanol.
lizing agents are mixed at their final concentrations Microbatch, though, can not be used for crystalliza-
at the start of the experiment; thus, in order to obtain tiontrialscontainingsmallvolatileorganicmolecules
crystals, supersaturationmustbeattaineduponmix- such as dioxane, phenol, or thymol since these
ing. Consequently, the volume and the composition molecules dissolve into the oil (Chayen, 1998). Such
of a trial remain constant. This is in contrast to substancesareonlyappliedinmicrobatchiftheexper-
all other crystallization methods which undergo a imenter aims to explore the effect of progressive
self-screening process until equilibrium is reached disappearance of these additives from the solution.
(Fig. 3.1). The stability of the batch is an important
benefit for conducting diagnostic studies on the pro-
3.4.3 Fine tuning of the conditions around
cess of crystal growth since the history of the sample
the screen
can be followed reliably. However, this benefit may
become a handicap in the case of screening for crys- Once a ‘hit’ has been found, the next step is to
tallization conditions since it is likely that the grad- conduct a finer screen around these conditions by
ual change of conditions (en route to equilibrium), varying the concentrations of protein, precipitant,
which takes place by the other methods, may be the addition of additives, etc. In most cases, more than
crucial factor for the formation of crystals (Chayen three different ingredients may be needed in each
et al., 1996; Chayen, 1997a; D’Arcy et al., 1996). trial, making it difficult to manually pipette all the
A modification of the original microbatch method ingredientsintoonedropdirectlyundertheoil. Thus
provides a means of simultaneously retaining the the procedure in Protocol 3.2 should be followed.