Page 57 - Macromolecular Crystallography
P. 57
46 MACROMOLECULAR CRYS TALLOGRAPHY
McPherson, 1999; Ducruix and Giegé, 1992; Ducruix involves mixing of protein and the crystallizing
and Giegé, 1999; Chayen et al., 1996). agents at conditions that aim to achieve supersatu-
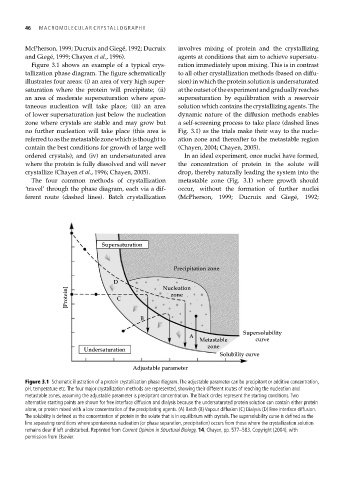
Figure 3.1 shows an example of a typical crys- ration immediately upon mixing. This is in contrast
tallization phase diagram. The figure schematically to all other crystallization methods (based on diffu-
illustrates four areas: (i) an area of very high super- sion) in which the protein solution is undersaturated
saturation where the protein will precipitate; (ii) at the outset of the experiment and gradually reaches
an area of moderate supersaturation where spon- supersaturation by equilibration with a reservoir
taneous nucleation will take place; (iii) an area solution which contains the crystallizing agents. The
of lower supersaturation just below the nucleation dynamic nature of the diffusion methods enables
zone where crystals are stable and may grow but a self-screening process to take place (dashed lines
no further nucleation will take place (this area is Fig. 3.1) as the trials make their way to the nucle-
referredtoasthemetastablezonewhichisthoughtto ation zone and thereafter to the metastable region
contain the best conditions for growth of large well (Chayen, 2004; Chayen, 2005).
ordered crystals); and (iv) an undersaturated area In an ideal experiment, once nuclei have formed,
where the protein is fully dissolved and will never the concentration of protein in the solute will
crystallize (Chayen et al., 1996; Chayen, 2005). drop, thereby naturally leading the system into the
The four common methods of crystallization metastable zone (Fig. 3.1) where growth should
‘travel’ through the phase diagram, each via a dif- occur, without the formation of further nuclei
ferent route (dashed lines). Batch crystallization (McPherson, 1999; Ducruix and Giegé, 1992;
Supersaturation
Precipitation zone
D Nucleation
[Protein] C zone
B
Supersolubility
A
Metastable curve
zone
Undersaturation
Solubility curve
Adjustable parameter
Figure 3.1 Schematic illustration of a protein crystallization phase diagram. The adjustable parameter can be precipitant or additive concentration,
pH, temperature etc. The four major crystallization methods are represented, showing their different routes of reaching the nucleation and
metastable zones, assuming the adjustable parameter is precipitant concentration. The black circles represent the starting conditions. Two
alternative starting points are shown for free interface diffusion and dialysis because the undersaturated protein solution can contain either protein
alone, or protein mixed with a low concentration of the precipitating agents. (A) Batch (B) Vapour diffusion (C) Dialysis (D) Free interface diffusion.
The solubility is defined as the concentration of protein in the solute that is in equilibrium with crystals. The supersolubility curve is defined as the
line separating conditions where spontaneous nucleation (or phase separation, precipitation) occurs from those where the crystallization solution
remains clear if left undisturbed. Reprinted from Current Opinion in Structural Biology, 14, Chayen, pp. 577–583, Copyright (2004), with
permission from Elsevier.