Page 72 - Macromolecular Crystallography
P. 72
FIRST ANALYSIS OF MACROMOLECULAR CRYS TALS 61
4.2.2.3 Transfer into an immiscible hydrocarbon and not crystalline ice, which would damage the
A third method for cryoprotecting macromolecular sample. Three types of cryogens are currently in
crystals involves replacing the solvent around the widespread use and these are freshly thawed liquid
crystal with a water immiscible hydrocarbon prior to propane (or ethane), liquid nitrogen, or cold gaseous
shock cooling. In a manner similar to the ‘quick-dip’ nitrogen (Garman and Schneider, 1997; Rodgers,
method, a loop-mounted crystal is passed through 1997; Garman, 2003).
a small drop of a hydrocarbon (e.g. Para tone-N,
dried paraffin oil, etc.). During this time, the aque- 4.3.1 Mounting a crystal in a fibre loop
ous mother liquor is sloughed-off as a result of being
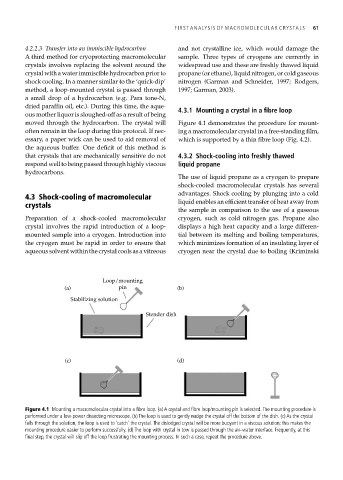
moved through the hydrocarbon. The crystal will Figure 4.1 demonstrates the procedure for mount-
often remain in the loop during this protocol. If nec- ing a macromolecular crystal in a free-standing film,
essary, a paper wick can be used to aid removal of which is supported by a thin fibre loop (Fig. 4.2).
the aqueous buffer. One deficit of this method is
that crystals that are mechanically sensitive do not 4.3.2 Shock-cooling into freshly thawed
respond well to being passed through highly viscous liquid propane
hydrocarbons.
The use of liquid propane as a cryogen to prepare
shock-cooled macromolecular crystals has several
advantages. Shock cooling by plunging into a cold
4.3 Shock-cooling of macromolecular
crystals liquid enables an efficient transfer of heat away from
the sample in comparison to the use of a gaseous
Preparation of a shock-cooled macromolecular cryogen, such as cold nitrogen gas. Propane also
crystal involves the rapid introduction of a loop- displays a high heat capacity and a large differen-
mounted sample into a cryogen. Introduction into tial between its melting and boiling temperatures,
the cryogen must be rapid in order to ensure that which minimizes formation of an insulating layer of
aqueous solvent within the crystal cools as a vitreous cryogen near the crystal due to boiling (Kriminski
Loop/mounting
(a) pin (b)
Stabilizing solution
Stender dish
(c) (d)
Figure 4.1 Mounting a macromolecular crystal into a fibre loop. (a) A crystal and fibre loop/mounting pin is selected. The mounting procedure is
performed under a low-power dissecting microscope. (b) The loop is used to gently nudge the crystal off the bottom of the dish. (c) As the crystal
falls through the solution, the loop is used to ‘catch’ the crystal. The dislodged crystal will be more buoyant in a viscous solution; this makes the
mounting procedure easier to perform successfully. (d) The loop with crystal in tow is passed through the air–water interface. Frequently, at this
final step, the crystal will slip off the loop frustrating the mounting process. In such a case, repeat the procedure above.