Page 75 - Macromolecular Crystallography
P. 75
64 MACROMOLECULAR CRYS TALLOGRAPHY
Liquid nitrogen
(a) nitrogen exhibits the lowest heat capacity in compar-
or
Liquid propane ison to liquid propane and liquid nitrogen and thus
gives rise to a slower rate of cooling (Kriminski et al.,
Cryovial
Liquid 2003). In some cases, this may prove to be an advan-
nitrogen bath tage. The efficiency and reproducibility of preparing
samples using this method can be highly dependent
Dewar on the type of cold stream being used, how it is being
flask
operated, and the dexterity of the operator. Finally,
(b) the requirement for a cold-stream to prepare sam-
ples precludes the flexibility of sample preparation
Loop mounted
crystal (e.g. the samples need to stable at room tempera-
ture, which is how most X-ray cameras are arranged)
found with the use of other cryogens.
4.4 Assembly of the mounted crystal
onto the X-ray diffraction camera
4.5 Storage and transport of
macromolecular crystals
TheavailabilityofintensesynchrotronX-radiationat
dedicated centres far from the investigator’s home
(c)
laboratory requires a reliable means of transport-
ing cryocooled macromolecular crystals. Commer-
cial shipping companies will accept for transport
properly prepared ‘dry shipping’ Dewar containers.
These containers have all the elements of a conven-
tional storage Dewar in addition to a material that
absorbs liquid nitrogen and can maintain cryogenic
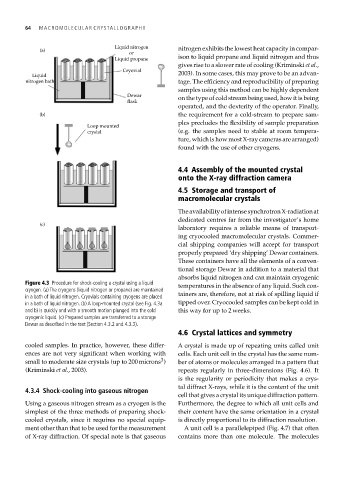
Figure 4.3 Procedure for shock-cooling a crystal using a liquid temperatures in the absence of any liquid. Such con-
cryogen. (a) The cryogens (liquid nitrogen or propane) are maintained
in a bath of liquid nitrogen. Cryovials containing cryogens are placed tainers are, therefore, not at risk of spilling liquid if
in a bath of liquid nitrogen. (b) A loop-mounted crystal (see Fig. 4.3a tipped over. Cryocooled samples can be kept cold in
and b) is quickly and with a smooth motion plunged into the cold this way for up to 2 weeks.
cryogenic liquid. (c) Prepared samples are transferred to a storage
Dewar as described in the text (Section 4.3.2 and 4.3.3).
4.6 Crystal lattices and symmetry
cooled samples. In practice, however, these differ- A crystal is made up of repeating units called unit
ences are not very significant when working with cells. Each unit cell in the crystal has the same num-
3
small to moderate size crystals (up to 200 microns ) ber of atoms or molecules arranged in a pattern that
(Kriminski et al., 2003). repeats regularly in three-dimensions (Fig. 4.6). It
is the regularity or periodicity that makes a crys-
tal diffract X-rays, while it is the content of the unit
4.3.4 Shock-cooling into gaseous nitrogen
cell that gives a crystal its unique diffraction pattern.
Using a gaseous nitrogen stream as a cryogen is the Furthermore, the degree to which all unit cells and
simplest of the three methods of preparing shock- their content have the same orientation in a crystal
cooled crystals, since it requires no special equip- is directly proportional to its diffraction resolution.
ment other than that to be used for the measurement A unit cell is a parallelepiped (Fig. 4.7) that often
of X-ray diffraction. Of special note is that gaseous contains more than one molecule. The molecules