Page 73 - Macromolecular Crystallography
P. 73
62 MACROMOLECULAR CRYS TALLOGRAPHY
et al., 2003). However, this method has some hardware requirements and the necessity to work
important disadvantages, which include additional with a flammable/explosive material. Finally, com-
mercial shipping of propane requires special paper-
work and handling due the associated hazards.
(a) (b) Thin film of
cryoprotecting
buffer
4.3.3 Shock-cooling into liquid nitrogen
The use of liquid nitrogen as a cryogen exploits its
liquid state to enable efficient heat transfer away
from the crystalline sample (as compared to gaseous
nitrogen), but without the specialized hardware
and safety concerns associated with use of liquid
propane. An additional advantage is that commer-
cial shipping of samples prepared in this way is
much less cumbersome since transport companies
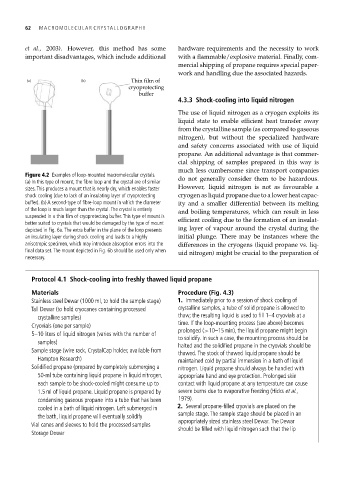
Figure 4.2 Examples of loop-mounted macromolecular crystals.
(a) In this type of mount, the fibre loop and the crystal are of similar do not generally consider them to be hazardous.
sizes. This produces a mount that is nearly dry, which enables faster However, liquid nitrogen is not as favourable a
shock cooling (due to lack of an insulating layer of cryoprotecting cryogen as liquid propane due to a lower heat capac-
buffer). (b) A second-type of fibre-loop mount in which the diameter ity and a smaller differential between its melting
of the loop is much larger than the crystal. The crystal is entirely and boiling temperatures, which can result in less
suspended in a thin film of cryoprotecting buffer. This type of mount is efficient cooling due to the formation of an insulat-
better suited to crystals that would be damaged by the type of mount
depicted in Fig. 6a. The extra buffer in the plane of the loop presents ing layer of vapour around the crystal during the
an insulating layer during shock cooling and leads to a highly initial plunge. There may be instances where the
anisotropic specimen, which may introduce absorption errors into the differences in the cryogens (liquid propane vs. liq-
final data set. The mount depicted in Fig. 6b should be used only when uid nitrogen) might be crucial to the preparation of
necessary.
Protocol 4.1 Shock-cooling into freshly thawed liquid propane
Materials Procedure (Fig. 4.3)
Stainless steel Dewar (1000 ml, to hold the sample stage) 1. Immediately prior to a session of shock cooling of
Tall Dewar (to hold cryocanes containing processed crystalline samples, a tube of solid propane is allowed to
crystalline samples) thaw; the resulting liquid is used to fill 1–4 cryovials at a
time. If the loop-mounting process (see above) becomes
Cryovials (one per sample)
prolonged (>10–15 min), the liquid propane might begin
5–10 liters of liquid nitrogen (varies with the number of
to solidify. In such a case, the mounting process should be
samples)
halted and the solidified propane in the cryovials should be
Sample stage (wire rack, CrystalCap holder, available from thawed. The stock of thawed liquid propane should be
Hampton Research) maintained cold by partial immersion in a bath of liquid
Solidified propane (prepared by completely submerging a nitrogen. Liquid propane should always be handled with
50-ml tube containing liquid propane in liquid nitrogen, appropriate hand and eye protection. Prolonged skin
each sample to be shock-cooled might consume up to contact with liquid propane at any temperature can cause
1.5 ml of liquid propane. Liquid propane is prepared by severe burns due to evaporative freezing (Hicks et al.,
condensing gaseous propane into a tube that has been 1979).
cooled in a bath of liquid nitrogen. Left submerged in 2. Several propane-filled cryovials are placed on the
sample stage. The sample stage should be placed in an
the bath, liquid propane will eventually solidify
appropriately sized stainless steel Dewar. The Dewar
Vial canes and sleeves to hold the processed samples
should be filled with liquid nitrogen such that the lip
Storage Dewar