Page 212 - Managing Global Warming
P. 212
Current and future nuclear power reactors and plants 173
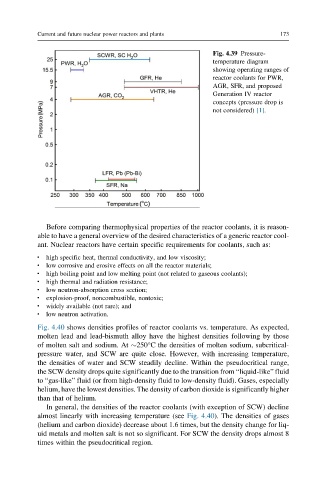
Fig. 4.39 Pressure-
temperature diagram
showing operating ranges of
reactor coolants for PWR,
AGR, SFR, and proposed
Generation IV reactor
concepts (pressure drop is
not considered) [1].
Before comparing thermophysical properties of the reactor coolants, it is reason-
able to have a general overview of the desired characteristics of a generic reactor cool-
ant. Nuclear reactors have certain specific requirements for coolants, such as:
l high specific heat, thermal conductivity, and low viscosity;
l low corrosive and erosive effects on all the reactor materials;
l high boiling point and low melting point (not related to gaseous coolants);
l high thermal and radiation resistance;
l low neutron-absorption cross section;
l explosion-proof, noncombustible, nontoxic;
l widely available (not rare); and
l low neutron activation.
Fig. 4.40 shows densities profiles of reactor coolants vs. temperature. As expected,
molten lead and lead-bismuth alloy have the highest densities following by those
of molten salt and sodium. At 250°C the densities of molten sodium, subcritical-
pressure water, and SCW are quite close. However, with increasing temperature,
the densities of water and SCW steadily decline. Within the pseudocritical range,
the SCW density drops quite significantly due to the transition from “liquid-like” fluid
to “gas-like” fluid (or from high-density fluid to low-density fluid). Gases, especially
helium, have the lowest densities. The density of carbon dioxide is significantly higher
than that of helium.
In general, the densities of the reactor coolants (with exception of SCW) decline
almost linearly with increasing temperature (see Fig. 4.40). The densities of gases
(helium and carbon dioxide) decrease about 1.6 times, but the density change for liq-
uid metals and molten salt is not so significant. For SCW the density drops almost 8
times within the pseudocritical region.