Page 215 - Managing Global Warming
P. 215
176 Managing Global Warming
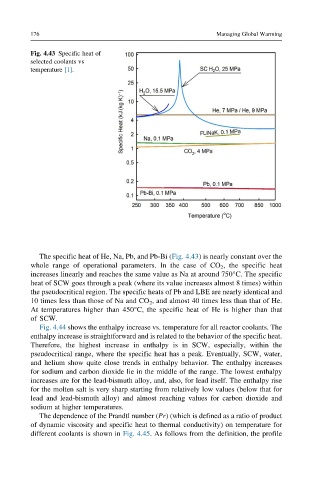
Fig. 4.43 Specific heat of
selected coolants vs
temperature [1].
The specific heat of He, Na, Pb, and Pb-Bi (Fig. 4.43) is nearly constant over the
whole range of operational parameters. In the case of CO 2 , the specific heat
increases linearly and reaches the same value as Na at around 750°C. The specific
heat of SCW goes through a peak (where its value increases almost 8 times) within
the pseudocritical region. The specific heats of Pb and LBE are nearly identical and
10 times less than those of Na and CO 2 , and almost 40 times less than that of He.
At temperatures higher than 450°C, the specific heat of He is higher than that
of SCW.
Fig. 4.44 shows the enthalpy increase vs. temperature for all reactor coolants. The
enthalpy increase is straightforward and is related to the behavior of the specific heat.
Therefore, the highest increase in enthalpy is in SCW, especially, within the
pseudocritical range, where the specific heat has a peak. Eventually, SCW, water,
and helium show quite close trends in enthalpy behavior. The enthalpy increases
for sodium and carbon dioxide lie in the middle of the range. The lowest enthalpy
increases are for the lead-bismuth alloy, and, also, for lead itself. The enthalpy rise
for the molten salt is very sharp starting from relatively low values (below that for
lead and lead-bismuth alloy) and almost reaching values for carbon dioxide and
sodium at higher temperatures.
The dependence of the Prandtl number (Pr) (which is defined as a ratio of product
of dynamic viscosity and specific heat to thermal conductivity) on temperature for
different coolants is shown in Fig. 4.45. As follows from the definition, the profile