Page 213 - Managing Global Warming
P. 213
174 Managing Global Warming
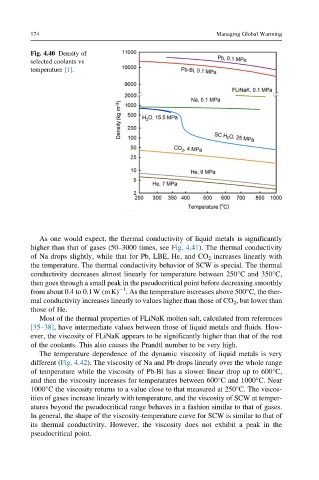
Fig. 4.40 Density of
selected coolants vs
temperature [1].
As one would expect, the thermal conductivity of liquid metals is significantly
higher than that of gases (50–3000 times, see Fig. 4.41). The thermal conductivity
of Na drops slightly, while that for Pb, LBE, He, and CO 2 increases linearly with
the temperature. The thermal conductivity behavior of SCW is special. The thermal
conductivity decreases almost linearly for temperature between 250°C and 350°C,
then goes through a small peak in the pseudocritical point before decreasing smoothly
1
from about 0.4 to 0.1W (mK) . As the temperature increases above 500°C, the ther-
mal conductivity increases linearly to values higher than those of CO 2 , but lower than
those of He.
Most of the thermal properties of FLiNaK molten salt, calculated from references
[35–38], have intermediate values between those of liquid metals and fluids. How-
ever, the viscosity of FLiNaK appears to be significantly higher than that of the rest
of the coolants. This also causes the Prandtl number to be very high.
The temperature dependence of the dynamic viscosity of liquid metals is very
different (Fig. 4.42). The viscosity of Na and Pb drops linearly over the whole range
of temperature while the viscosity of Pb-Bi has a slower linear drop up to 600°C,
and then the viscosity increases for temperatures between 600°C and 1000°C. Near
1000°C the viscosity returns to a value close to that measured at 250°C. The viscos-
ities of gases increase linearly with temperature, and the viscosity of SCW at temper-
atures beyond the pseudocritical range behaves in a fashion similar to that of gases.
In general, the shape of the viscosity-temperature curve for SCW is similar to that of
its thermal conductivity. However, the viscosity does not exhibit a peak in the
pseudocritical point.