Page 218 - Managing Global Warming
P. 218
Current and future nuclear power reactors and plants 179
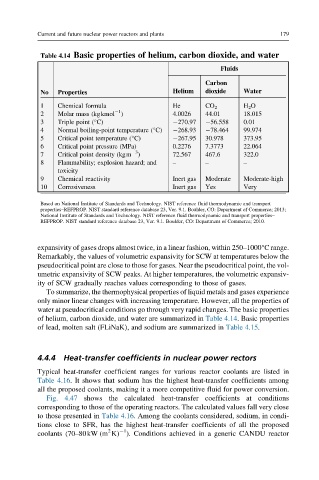
Table 4.14 Basic properties of helium, carbon dioxide, and water
Fluids
Carbon
Helium dioxide Water
No Properties
1 Chemical formula He CO 2 H 2 O
1
2 Molar mass (kgkmol ) 4.0026 44.01 18.015
3 Triple point (°C) 270.97 56.558 0.01
4 Normal boiling-point temperature (°C) 268.93 78.464 99.974
5 Critical point temperature (°C) 267.95 30.978 373.95
6 Critical point pressure (MPa) 0.2276 7.3773 22.064
3
7 Critical point density (kgm ) 72.567 467.6 322.0
8 Flammability; explosion hazard; and – – –
toxicity
9 Chemical reactivity Inert gas Moderate Moderate-high
10 Corrosiveness Inert gas Yes Very
Based on National Institute of Standards and Technology. NIST reference fluid thermodynamic and transport
properties–REFPROP. NIST standard reference database 23, Ver. 9.1. Boulder, CO: Department of Commerce; 2013;
National Institute of Standards and Technology. NIST reference fluid thermodynamic and transport properties–
REFPROP. NIST standard reference database 23, Ver. 9.1. Boulder, CO: Department of Commerce; 2010.
expansivity of gases drops almost twice, in a linear fashion, within 250–1000°C range.
Remarkably, the values of volumetric expansivity for SCW at temperatures below the
pseudocritical point are close to those for gases. Near the pseudocritical point, the vol-
umetric expansivity of SCW peaks. At higher temperatures, the volumetric expansiv-
ity of SCW gradually reaches values corresponding to those of gases.
To summarize, the thermophysical properties of liquid metals and gases experience
only minor linear changes with increasing temperature. However, all the properties of
water at pseudocritical conditions go through very rapid changes. The basic properties
of helium, carbon dioxide, and water are summarized in Table 4.14. Basic properties
of lead, molten salt (FLiNaK), and sodium are summarized in Table 4.15.
4.4.4 Heat-transfer coefficients in nuclear power rectors
Typical heat-transfer coefficient ranges for various reactor coolants are listed in
Table 4.16. It shows that sodium has the highest heat-transfer coefficients among
all the proposed coolants, making it a more competitive fluid for power conversion.
Fig. 4.47 shows the calculated heat-transfer coefficients at conditions
corresponding to those of the operating reactors. The calculated values fall very close
to those presented in Table 4.16. Among the coolants considered, sodium, in condi-
tions close to SFR, has the highest heat-transfer coefficients of all the proposed
2 1
coolants (70–80kW (m K) ). Conditions achieved in a generic CANDU reactor