Page 256 -
P. 256
CONCLUSIONS 245
IND = Investigational
FDA time
Sponsor/FDA IRB = Institutional
New Drug
meetings Review Boards
Industry time NDA = New Drug
Application
Pre-clinical
research Clinical studies Nda Review
PHASE I
Synthesis &
purification
PHASE II
PHASE III
Animal
testing
SHORT TERM
LONG TERM
IRBs
IND NDA
submitted submitted Sponsor
answers
Review review
decision QUESTIONS
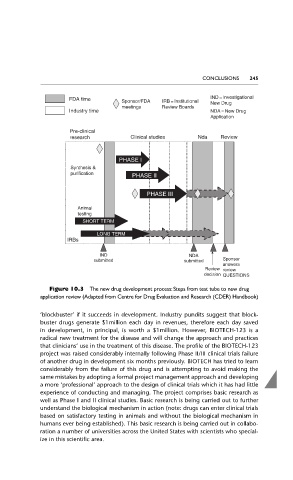
Figure 10.3 The new drug development process: Steps from test tube to new drug
application review (Adapted from Centre for Drug Evaluation and Research (CDER) Handbook)
‘blockbuster’ if it succeeds in development. Industry pundits suggest that block-
buster drugs generate $1million each day in revenues, therefore each day saved
in development, in principal, is worth a $1million. However, BIOTECH-123 is a
radical new treatment for the disease and will change the approach and practices
that clinicians’ use in the treatment of this disease. The profile of the BIOTECH-123
project was raised considerably internally following Phase II/III clinical trials failure
of another drug in development six months previously. BIOTECH has tried to learn
considerably from the failure of this drug and is attempting to avoid making the
same mistakes by adopting a formal project management approach and developing
a more ‘professional’ approach to the design of clinical trials which it has had little
experience of conducting and managing. The project comprises basic research as
well as Phase I and II clinical studies. Basic research is being carried out to further
understand the biological mechanism in action (note: drugs can enter clinical trials
based on satisfactory testing in animals and without the biological mechanism in
humans ever being established). This basic research is being carried out in collabo-
ration a number of universities across the United States with scientists who special-
ize in this scientific area.
6/5/09 7:21:37 AM
9780230_522015_11_cha10.indd 245
9780230_522015_11_cha10.indd 245 6/5/09 7:21:37 AM