Page 127 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 127
Chapter 4 Metal Alloys: Their Structure and Strengthening by Heat Treatment
" 500 A 300
Li’
3 ii’
.Q 400 5 200
`=7= 300 ‘<7> 100
2 E
'G .Q
C >-
ii’ 200 0
0 25 50 75 100 Ni0rZn 0 25 50 75 100 NiorZn
100 75 50 25 0 Cu 100 75 50 25 0 Cu
Composition (%) Composition (%)
120 A 70
g
A E Zinc
Ei 100 3 60
C
-f -- 50
§ 80 5,
_E 5 40
6° §» so
iz”
O
40 E 20
0 25 50 75 100 NiorZn 0 25 50 75 100 NiorZn
100 75 50 25 0 Cu 100 75 50 25 0 Cu
Composition (%) Composition (%)
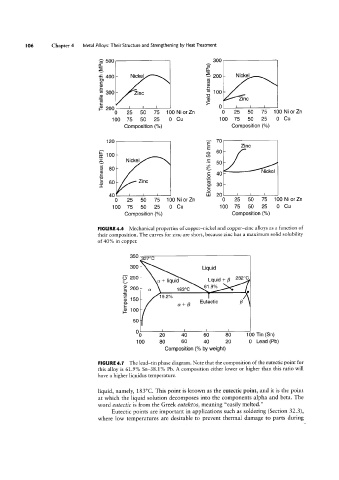
FIGURE 4.6 Mechanical properties of copper-nickel and copper-zinc alloys as a function of
their composition. The curves for zinc are short, because zinc has a maximum solid solubility
of 40% in copper.
350 32706
300 _ Liquid
Sl 250 ` of + iiquid Liquid + B 232%
g 200 _ a 183OC 61.9%
E _ 19.2%
él 150 Ol + B Eutectic B
.9 100 -
50
O
0 20 40 60 80 100 Tin (Sn)
100 80 60 40 20 0 Lead (Pb)
Composition (% by weight)
FIGURE 4.1 The lead-tin phase diagram. Note that the composition of the eutectic point for
this alloy is 61.9% Sn-38.1% Pb. A composition either lower or higher than this ratio will
have a higher liquidus temperature.
liquid, namely, 183°C. This point is known as the eutectic point, and it is the point
at which the liquid solution decomposes into the components alpha and beta. The
Word eutectic is from the Greek eute/etos, meaning “easily melted.”
Eutectic points are important in applications such as soldering (Section 323),
where low temperatures are desirable to prevent thermal damage to parts during