Page 128 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 128
Section 4.4 The Iron Carbon System
joining. Although there are various types of solders, tin-lead solders are commonly
used for general applications; they have compositions ranging from 5% Pb-95 % Sn
to 70% Pb-30% Sn. Each composition has its own melting point.
4.4 The Iron-Carbon System
Steels and cast irons are represented by the iron-carbon binary system. Commercially
pure iron contains up to 0.008% C, steels up to 2.11% C, and cast irons up to
6.67% C, although most cast irons contain less than 4.5% C. In this section the
iron-carbon system is described, including the techniques employed to evaluate and
modify the properties of these important materials for specific applications.
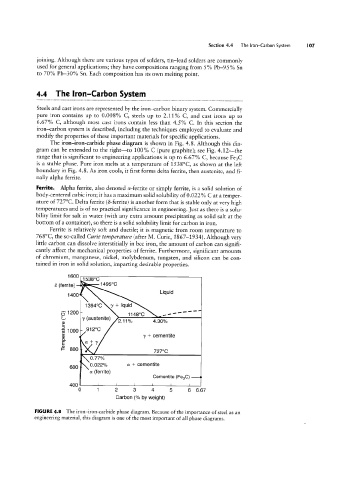
The iron-iron-carbide phase diagram is shown in Fig. 4.8, Although this dia-
gram can be extended to the right-to 100% C (pure graphite); see Fig. 4.12~the
range that is significant to engineering applications is up to 6.67% C, because Fe3C
is a stable phase. Pure iron melts at a temperature of 1538°C, as shown at the left
boundary in Fig. 4.8. As iron cools, it first forms delta ferrite, then austenite, and fi-
nally alpha ferrite.
Ferrite. Alpha ferrite, also denoted a-ferrite or simply ferrite, is a solid solution of
body-centered cubic iron; it has a maximum solid solubility of 0.022% C at a temper-
ature of 727°C. Delta ferrite (5-ferrite) is another form that is stable only at very high
temperatures and is of no practical significance in engineering. just as there is a solu-
bility limit for salt in Water (With any extra amount precipitating as solid salt at the
bottom of a container), so there is a solid solubility limit for carbon in iron.
Ferrite is relatively soft and ductile; it is magnetic from room temperature to
768°C, the so-called Curie temperature (after M. Curie, 1867-1934). Although very
little carbon can dissolve interstitially in bcc iron, the amount of carbon can signifi-
cantly affect the mechanical properties of ferrite. Furthermore, significant amounts
of chromium, manganese, nickel, molybdenum, tungsten, and silicon can be con-
tained in iron in solid solution, imparting desirable properties.
160° 1538°C O
6 (ferrite) 1495 C
1400 Liquid
1394°C 1' + liquid
,»*"""'
§l2O°` .1 114s°C
5 I’ (a“S‘e'“' el 2.11% 4.30%
1% 1000 - 9I2°C
3, y + cementite
E' oz -I- 'y
19 soo 727.0
0.77%
600 0_022% a + cementite
CY (ferrite) Cementite (Fe3C) -¢
O 1 2 3 4 5 6 6.67
Carbon (% by weight)
FIGURE 4.8 The iron-iron-carbide phase diagram. Because of the importance of steel as an
engineering material, this diagram is one of the most important of all phase diagrams.