Page 130 - 04. Subyek Engineering Materials - Manufacturing, Engineering and Technology SI 6th Edition - Serope Kalpakjian, Stephen Schmid (2009)
P. 130
Section 4.5 The lron-Iron-carbide Phase Diagram and the Development of Microstructures in Steels |09
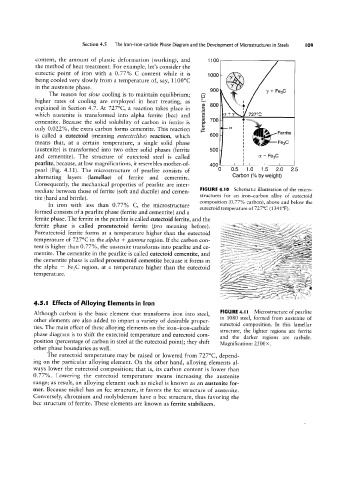
content, the amount of plastic deformation (working), and 1100
mentite. The cementite in the pearlite is called eutectoid cementite, and
the method of heat treatment. For example, let’s consider the
eutectic point of iron with a 0.77% C content while it is 1000 -
being cooled very slowly from a temperature of, say, 1100°C
in the austenite phase.
A 900 y + Feac
The reason for slow cooling is to maintain equilibrium; 9 800 4
S9
higher rates of cooling are employed in heat treating, as
3
explained in Section 4.7. At 727°C, a reaction takes place in and the
3 700 J ¢
which austenite is transformed into alpha ferrite (bcc) and E a + 1/ 727°C
cementite. Because the solid solubility of carbon in ferrite is
only 0.022%, the extra carbon forms cementite. This reaction E ._ 0. 3'
l' 600 _ Ferrite
is called a eutectoid (meaning eutecticli/ze) reaction, which
means that, at a certain temperature, a single solid phase F93C
(austenite) is transformed into two other solid phases (ferrite 500 1
and cementite). The structure of eutectoid steel is called U 'l' Fegc
pearlite, because, at low magnifications, it resembles mother-of-
pearl (Fig. 4.11). The microstructure of pearlite consists of 0 0.5 1.0 1.5 2.0 2.5
alternating layers (lamellae) of ferrite and cementite. Carbon (% by weight)
Consequently, the mechanical properties of pearlite are inter-
FIGURE 4.I0 Schematic illustration of the micro-
mediate between those of ferrite (soft and ductile) and cemen-
structures for an iron-carbon alloy of eutectoid
tite (hard and brittle).
composition (0.77% carbon), above and below the
In iron with less than 0.77% C, the microstructure
eutectoid temperature of 727°C (1341°F).
formed consists of a pearlite phase (ferrite and cementite) and a
ferrite phase. The ferrite in the pearlite is called eutectoid ferrite,
ferrite phase is called proeutectoid ferrite (pro meaning before).
Poreutectoid ferrite forms at a temperature higher than the eutectoid /'
temperature of 727°C in the alpha -l- gamma region. If the carbon con- °” »-»~a., X
w-=¢:=,, --
“°
tent is higher than 0.77%, the austenite transforms into pearlite and ce- ,.._ ~ """“““" TZ# .3 ma-- ~
,
the cementite phase is called proeutectoid cementite because it forms in ,.._.
the alpha + Fe3C region, at a temperature higher than the eutectoid
temperature. 5' ..s,..;_‘_‘ ~»-~f-..»<....a,,,,Wa»a.~._'_.`,,,,..v.“>»‘ /ff-=a;,,,»
if ..1»1 aaa
4.5.I Effects of Alloying Elements in Iron
FIGURE 4.l Microstructure of pearlite
Although carbon is the basic element that transforms iron into steel, I
in 1080 steel, formed from austenite of
other elements are also added to impart a Variety of desirable proper-
eutectoid composition. In this lamellar
ties. The main effect of these alloying elements on the iron-iron-carbide
structure, the lighter regions are ferrite
phase diagram is to shift the eutectoid temperature and eutectoid com-
and the darker regions are carbide.
position (percentage of carbon in steel at the eutectoid point); they shift
Magnification: 2500><.
other phase boundaries as well.
The eutectoid temperature may be raised or lowered from 727°C, depend-
ing on the particular alloying element. On the other hand, alloying elements al-
ways lower the eutectoid composition; that is, its carbon content is lower than
0.77%. Lowering the eutectoid temperature means increasing the austenite
range; as result, an alloying element such as nickel is known as an austenite for-
mer. Because nickel has an fcc structure, it favors the fcc structure of austenite.
Conversely, chromium and molybdenum have a bcc structure, thus favoring the
bcc structure of ferrite. These elements are known as ferrite stabilizers.