Page 259 - Materials Science and Engineering An Introduction
P. 259
7.9 Solid-Solution Strengthening • 231
Small-angle grain boundaries (Section 4.6) are not effective in interfering with the
slip process because of the slight crystallographic misalignment across the boundary.
However, twin boundaries (Section 4.6) effectively block slip and increase the strength
of the material. Boundaries between two different phases are also impediments to move-
ments of dislocations; this is important in the strengthening of more complex alloys. The
sizes and shapes of the constituent phases significantly affect the mechanical properties
of multiphase alloys; these are the topics of discussion in Sections 10.7, 10.8, and 16.1.
7.9 SOLID-SOLUTION STRENGTHENING
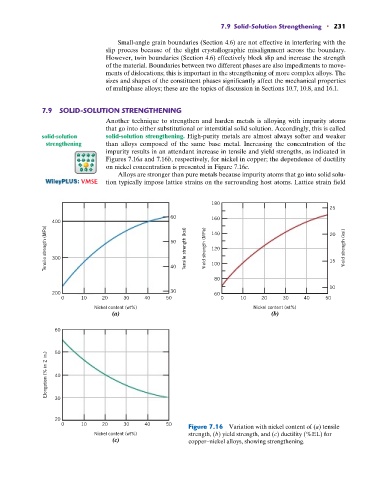
Another technique to strengthen and harden metals is alloying with impurity atoms
that go into either substitutional or interstitial solid solution. Accordingly, this is called
solid-solution solid-solution strengthening. High-purity metals are almost always softer and weaker
strengthening than alloys composed of the same base metal. Increasing the concentration of the
impurity results in an attendant increase in tensile and yield strengths, as indicated in
Figures 7.16a and 7.16b, respectively, for nickel in copper; the dependence of ductility
on nickel concentration is presented in Figure 7.16c.
Alloys are stronger than pure metals because impurity atoms that go into solid solu-
: VMSE tion typically impose lattice strains on the surrounding host atoms. Lattice strain field
180
25
60 160
400 140 20
Tensile strength (MPa) 300 50 Tensile strength (ksi) Yield strength (MPa) 120 15 Yield strength (ksi)
100
40
80
10
30
200 60
0 10 20 30 40 50 0 10 20 30 40 50
Nickel content (wt%) Nickel content (wt%)
(a) (b)
60
Elongation (% in 2 in.) 50
40
30
20
0 10 20 30 40 50 Figure 7.16 Variation with nickel content of (a) tensile
Nickel content (wt%) strength, (b) yield strength, and (c) ductility (%EL) for
(c) copper–nickel alloys, showing strengthening.