Page 327 - Materials Science and Engineering An Introduction
P. 327
9.2 Solubility Limit • 299
which are also common terms, were defined in Section 4.3. Another term used in
system this context is system, which has two meanings. System may refer to a specific body
of material under consideration (e.g., a ladle of molten steel); or it may relate to the
series of possible alloys consisting of the same components but without regard to
alloy composition (e.g., the iron–carbon system).
The concept of a solid solution was introduced in Section 4.3. To review, a solid
solution consists of atoms of at least two different types; the solute atoms occupy either
substitutional or interstitial positions in the solvent lattice, and the crystal structure of
the solvent is maintained.
9.2 SOLUBILITY LIMIT
For many alloy systems and at some specific temperature, there is a maximum concen-
tration of solute atoms that may dissolve in the solvent to form a solid solution; this is
solubility limit called a solubility limit. The addition of solute in excess of this solubility limit results in
the formation of another solid solution or compound that has a distinctly different com-
position. To illustrate this concept, consider the sugar–water (C 12 H 22 O 11 –H 2 O) system.
Initially, as sugar is added to water, a sugar–water solution or syrup forms. As more
sugar is introduced, the solution becomes more concentrated, until the solubility limit is
reached or the solution becomes saturated with sugar. At this time, the solution is not
capable of dissolving any more sugar, and further additions simply settle to the bottom
of the container. Thus, the system now consists of two separate substances: a sugar–water
syrup liquid solution and solid crystals of undissolved sugar.
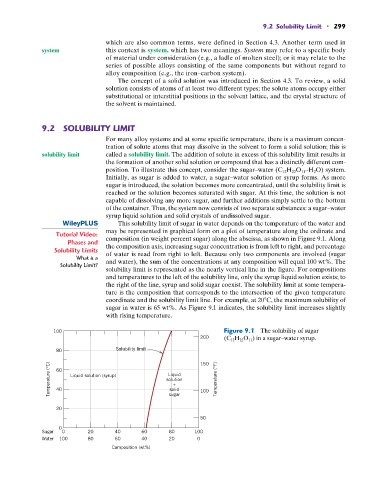
This solubility limit of sugar in water depends on the temperature of the water and
may be represented in graphical form on a plot of temperature along the ordinate and
Tutorial Video: composition (in weight percent sugar) along the abscissa, as shown in Figure 9.1. Along
Phases and
Solubility Limits the composition axis, increasing sugar concentration is from left to right, and percentage
of water is read from right to left. Because only two components are involved (sugar
What is a and water), the sum of the concentrations at any composition will equal 100 wt%. The
Solubility Limit?
solubility limit is represented as the nearly vertical line in the figure. For compositions
and temperatures to the left of the solubility line, only the syrup liquid solution exists; to
the right of the line, syrup and solid sugar coexist. The solubility limit at some tempera-
ture is the composition that corresponds to the intersection of the given temperature
coordinate and the solubility limit line. For example, at 20 C, the maximum solubility of
sugar in water is 65 wt%. As Figure 9.1 indicates, the solubility limit increases slightly
with rising temperature.
100 Figure 9.1 The solubility of sugar
200 (C 12 H 22 O 11 ) in a sugar–water syrup.
80 Solubility limit 150
Temperature (°C) 60 Liquid solution (syrup) solution 100 Temperature (°F)
Liquid
+
solid
40
20 sugar
50
0
Sugar 0 20 40 60 80 100
Water 100 80 60 40 20 0
Composition (wt%)