Page 52 - Materials Science and Engineering An Introduction
P. 52
24 • Chapter 2 / Atomic Structure and Interatomic Bonding
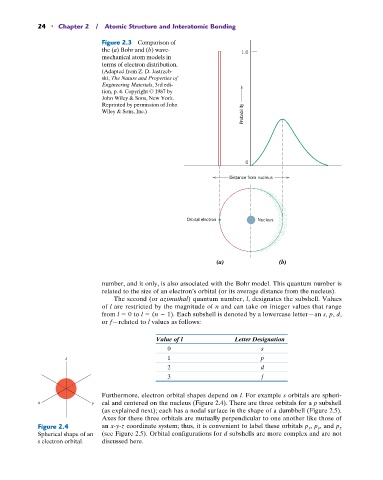
Figure 2.3 Comparison of
the (a) Bohr and (b) wave- 1.0
mechanical atom models in
terms of electron distribution.
(Adapted from Z. D. Jastrzeb-
ski, The Nature and Properties of
Engineering Materials, 3rd edi-
tion, p. 4. Copyright © 1987 by
John Wiley & Sons, New York.
Reprinted by permission of John
Probability
Wiley & Sons, Inc.)
0
Distance from nucleus
Orbital electron Nucleus
(a) (b)
number, and it only, is also associated with the Bohr model. This quantum number is
related to the size of an electron’s orbital (or its average distance from the nucleus).
The second (or azimuthal) quantum number, l, designates the subshell. Values
of l are restricted by the magnitude of n and can take on integer values that range
from l 0 to l (n 1). Each subshell is denoted by a lowercase letter—an s, p, d,
or f—related to l values as follows:
Value of l Letter Designation
0 s
z 1 p
2 d
3 f
Furthermore, electron orbital shapes depend on l. For example s orbitals are spheri-
x y cal and centered on the nucleus (Figure 2.4). There are three orbitals for a p subshell
(as explained next); each has a nodal surface in the shape of a dumbbell (Figure 2.5).
Axes for these three orbitals are mutually perpendicular to one another like those of
Figure 2.4 an x-y-z coordinate system; thus, it is convenient to label these orbitals p x , p y , and p z
Spherical shape of an (see Figure 2.5). Orbital configurations for d subshells are more complex and are not
s electron orbital. discussed here.