Page 53 - Materials Science and Engineering An Introduction
P. 53
2.3 Electrons in Atoms • 25
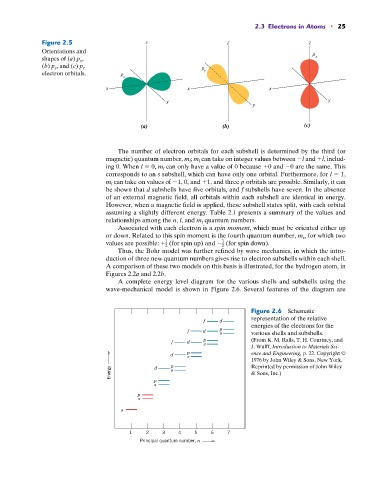
Figure 2.5 z z z
Orientations and p
shapes of (a) p x , z
(b) p y , and (c) p z p
electron orbitals. p y
x
x x x
y y
y
(a) (b) (c)
The number of electron orbitals for each subshell is determined by the third (or
magnetic) quantum number, m l ; m l can take on integer values between l and l, includ-
ing 0. When l 0, m l can only have a value of 0 because 0 and 0 are the same. This
corresponds to an s subshell, which can have only one orbital. Furthermore, for l 1,
m l can take on values of 1, 0, and 1, and three p orbitals are possible. Similarly, it can
be shown that d subshells have five orbitals, and f subshells have seven. In the absence
of an external magnetic field, all orbitals within each subshell are identical in energy.
However, when a magnetic field is applied, these subshell states split, with each orbital
assuming a slightly different energy. Table 2.1 presents a summary of the values and
relationships among the n, l, and m l quantum numbers.
Associated with each electron is a spin moment, which must be oriented either up
or down. Related to this spin moment is the fourth quantum number, m s , for which two
1
1
values are possible: + (for spin up) and - (for spin down).
2
2
Thus, the Bohr model was further refined by wave mechanics, in which the intro-
duction of three new quantum numbers gives rise to electron subshells within each shell.
A comparison of these two models on this basis is illustrated, for the hydrogen atom, in
Figures 2.2a and 2.2b.
A complete energy level diagram for the various shells and subshells using the
wave-mechanical model is shown in Figure 2.6. Several features of the diagram are
Figure 2.6 Schematic
representation of the relative
f d
energies of the electrons for the
p
f d
s various shells and subshells.
p (From K. M. Ralls, T. H. Courtney, and
f d
s
J. Wulff, Introduction to Materials Sci-
p ence and Engineering, p. 22. Copyright ©
d
s
1976 by John Wiley & Sons, New York.
p Reprinted by permission of John Wiley
Energy s & Sons, Inc.)
d
p
s
p
s
s
1 2 3 4 5 6 7
Principal quantum number, n