Page 58 - Materials Science and Engineering An Introduction
P. 58
30 • Chapter 2 / Atomic Structure and Interatomic Bonding
Atomic Bonding in Solids
2.5 BONDING FORCES AND ENERGIES
An understanding of many of the physical properties of materials is enhanced by a
knowledge of the interatomic forces that bind the atoms together. Perhaps the principles
of atomic bonding are best illustrated by considering how two isolated atoms interact as
they are brought close together from an infinite separation. At large distances, interac-
tions are negligible because the atoms are too far apart to have an influence on each
other; however, at small separation distances, each atom exerts forces on the others.
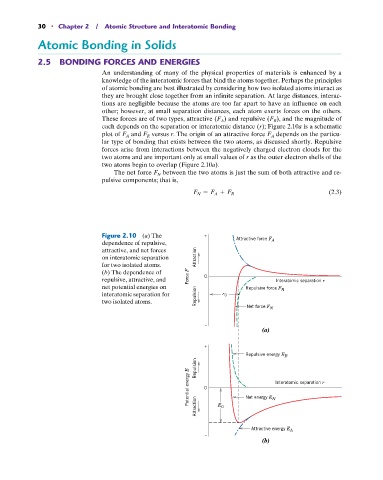
These forces are of two types, attractive (F A ) and repulsive (F R ), and the magnitude of
each depends on the separation or interatomic distance (r); Figure 2.10a is a schematic
plot of F A and F R versus r. The origin of an attractive force F A depends on the particu-
lar type of bonding that exists between the two atoms, as discussed shortly. Repulsive
forces arise from interactions between the negatively charged electron clouds for the
two atoms and are important only at small values of r as the outer electron shells of the
two atoms begin to overlap (Figure 2.10a).
The net force F N between the two atoms is just the sum of both attractive and re-
pulsive components; that is,
F N = F A + F R (2.3)
Figure 2.10 (a) The + Attractive force F
dependence of repulsive, A
attractive, and net forces
on interatomic separation Attraction
for two isolated atoms.
(b) The dependence of
repulsive, attractive, and Force F 0 Interatomic separation r
net potential energies on Repulsive force F R
interatomic separation for Repulsion r 0
two isolated atoms. Net force F N
–
(a)
+
Repulsive energy E R
Repulsion
Potential energy E Attraction 0 E 0 Net energy E N Interatomic separation r
Attractive energy E A
–
(b)