Page 55 - Materials Science and Engineering An Introduction
P. 55
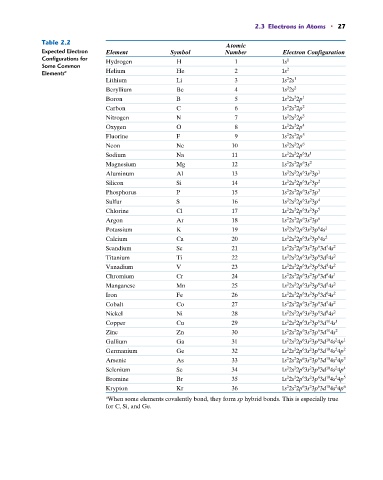
2.3 Electrons in Atoms • 27
Table 2.2
Atomic
Expected Electron Element Symbol Number Electron Configuration
Configurations for Hydrogen H 1 1s 1
Some Common 2
Elements a Helium He 2 1s
2
Lithium Li 3 1s 2s 1
2
Beryllium Be 4 1s 2s 2
2
2
Boron B 5 1s 2s 2p 1
2
2
Carbon C 6 1s 2s 2p 2
2
2
Nitrogen N 7 1s 2s 2p 3
2
2
Oxygen O 8 1s 2s 2p 4
2
2
Fluorine F 9 1s 2s 2p 5
2
2
Neon Ne 10 1s 2s 2p 6
2
2
6
Sodium Na 11 1s 2s 2p 3s 1
6
2
2
Magnesium Mg 12 1s 2s 2p 3s 2
2
2
2
6
Aluminum Al 13 1s 2s 2p 3s 3p 1
2
2
2
6
Silicon Si 14 1s 2s 2p 3s 3p 2
2
6
2
2
Phosphorus P 15 1s 2s 2p 3s 3p 3
2
2
2
6
Sulfur S 16 1s 2s 2p 3s 3p 4
2
2
6
2
Chlorine Cl 17 1s 2s 2p 3s 3p 5
2
2
2
6
Argon Ar 18 1s 2s 2p 3s 3p 6
6
6
2
2
2
Potassium K 19 1s 2s 2p 3s 3p 4s 1
2
6
6
2
2
Calcium Ca 20 1s 2s 2p 3s 3p 4s 2
2
6
2
2
1
6
Scandium Sc 21 1s 2s 2p 3s 3p 3d 4s 2
6
2
2
2
2
6
Titanium Ti 22 1s 2s 2p 3s 3p 3d 4s 2
2
2
3
2
6
6
Vanadium V 23 1s 2s 2p 3s 3p 3d 4s 2
6
6
2
5
2
2
Chromium Cr 24 1s 2s 2p 3s 3p 3d 4s 1
6
5
6
2
2
2
Manganese Mn 25 1s 2s 2p 3s 3p 3d 4s 2
2
2
6
6
2
6
Iron Fe 26 1s 2s 2p 3s 3p 3d 4s 2
6
6
2
7
2
2
Cobalt Co 27 1s 2s 2p 3s 3p 3d 4s 2
8
2
6
2
2
6
Nickel Ni 28 1s 2s 2p 3s 3p 3d 4s 2
6
10
6
2
2
2
Copper Cu 29 1s 2s 2p 3s 3p 3d 4s 1
6
2
2
10
2
6
Zinc Zn 30 1s 2s 2p 3s 3p 3d 4s 2
2
2
10
2
6
6
2
Gallium Ga 31 1s 2s 2p 3s 3p 3d 4s 4p 1
6
6
2
10
2
2
2
Germanium Ge 32 1s 2s 2p 3s 3p 3d 4s 4p 2
10
2
2
2
2
6
6
Arsenic As 33 1s 2s 2p 3s 3p 3d 4s 4p 3
2
2
10
2
6
2
6
Selenium Se 34 1s 2s 2p 3s 3p 3d 4s 4p 4
6
6
2
2
2
10
2
Bromine Br 35 1s 2s 2p 3s 3p 3d 4s 4p 5
2
2
6
10
2
2
6
Krypton Kr 36 1s 2s 2p 3s 3p 3d 4s 4p 6
a When some elements covalently bond, they form sp hybrid bonds. This is especially true
for C, Si, and Ge.