Page 132 - Materials Chemistry, Second Edition
P. 132
119
2.4. The Amorphous State
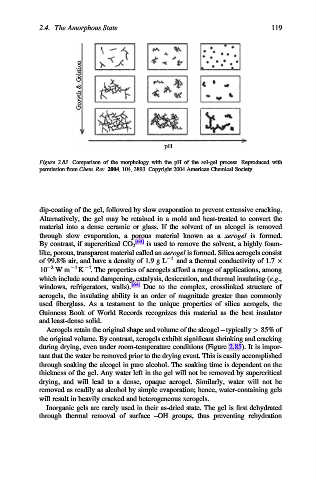
Growth & Gelation
pH
Figure 2.83. Comparison of the morphology with the pH of the sol-gel process. Reproduced with
permission from Chem. Rev. 2004, 104, 3893. Copyright 2004 American Chemical Society.
dip-coating of the gel, followed by slow evaporation to prevent extensive cracking.
Alternatively, the gel may be retained in a mold and heat-treated to convert the
material into a dense ceramic or glass. If the solvent of an alcogel is removed
through slow evaporation, a porous material known as a xerogel is formed.
[63]
By contrast, if supercritical CO 2 is used to remove the solvent, a highly foam-
like, porous, transparent material called an aerogel is formed. Silica aerogels consist
1
of 99.8% air, and have a density of 1.9 g L and a thermal conductivity of 1.7
1
10 2 Wm 1 K . The properties of aerogels afford a range of applications, among
which include sound dampening, catalysis, desiccation, and thermal insulating (e.g.,
windows, refrigerators, walls). [64] Due to the complex, crosslinked structure of
aerogels, the insulating ability is an order of magnitude greater than commonly
used fiberglass. As a testament to the unique properties of silica aerogels, the
Guinness Book of World Records recognizes this material as the best insulator
and least-dense solid.
Aerogels retain the original shape and volume of the alcogel – typically > 85% of
the original volume. By contrast, xerogels exhibit significant shrinking and cracking
during drying, even under room-temperature conditions (Figure 2.85). It is impor-
tant that the water be removed prior to the drying event. This is easily accomplished
through soaking the alcogel in pure alcohol. The soaking time is dependent on the
thickness of the gel. Any water left in the gel will not be removed by supercritical
drying, and will lead to a dense, opaque aerogel. Similarly, water will not be
removed as readily as alcohol by simple evaporation; hence, water-containing gels
will result in heavily cracked and heterogeneous xerogels.
Inorganic gels are rarely used in their as-dried state. The gel is first dehydrated
through thermal removal of surface –OH groups, thus preventing rehydration