Page 134 - Materials Chemistry, Second Edition
P. 134
121
2.4. The Amorphous State
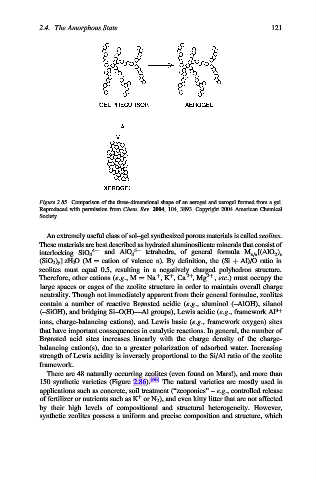
Figure 2.85. Comparison of the three-dimensional shape of an aerogel and xerogel formed from a gel.
Reproduced with permission from Chem. Rev. 2004, 104, 3893. Copyright 2004 American Chemical
Society.
An extremely useful class of sol–gel synthesized porous materials is called zeolites.
These materials are best described as hydrated aluminosilicate minerals that consist of
4 5
interlocking SiO 4 and AlO 4 tetrahedra, of general formula M x/n [(AlO 2 ) x
.
(SiO 2 ) y ] zH 2 O(M ¼ cation of valence n). By definition, the (Si þ Al)/O ratio in
zeolites must equal 0.5, resulting in a negatively charged polyhedron structure.
þ
þ
Therefore, other cations (e.g.,M ¼ Na ,K ,Ca ,Mg , etc.) must occupy the
2þ
2þ
large spaces or cages of the zeolite structure in order to maintain overall charge
neutrality. Though not immediately apparent from their general formulae, zeolites
contain a number of reactive Brønsted acidic (e.g., aluminol (–AlOH), silanol
(–SiOH), and bridging Si–O(H)—Al groups), Lewis acidic (e.g., framework Al 3þ
ions, charge-balancing cations), and Lewis basic (e.g., framework oxygen) sites
that have important consequences in catalytic reactions. In general, the number of
Brønsted acid sites increases linearly with the charge density of the charge-
balancing cation(s), due to a greater polarization of adsorbed water. Increasing
strength of Lewis acidity is inversely proportional to the Si/Al ratio of the zeolite
framework.
There are 48 naturally occurring zeolites (even found on Mars!), and more than
150 synthetic varieties (Figure 2.86). [66] The natural varieties are mostly used in
applications such as concrete, soil treatment (“zeoponics” – e.g., controlled release
þ
of fertilizer or nutrients such as K or N 2 ), and even kitty litter that are not affected
by their high levels of compositional and structural heterogeneity. However,
synthetic zeolites possess a uniform and precise composition and structure, which