Page 131 - Materials Chemistry, Second Edition
P. 131
118 2 Solid-State Chemistry
+
+ fast Si O R
(RO) Si ÖR + H (RO) 3
3
H
‡ +
RO OR ROH, H
+ H
(RO) Si O R (S 2) R
N
3
ÖH 2
O Si O (RO) Si OH
3
H
H H
OR
+
‡ ROH, H
(RO) Si OH RO OR H
3
+ slow (RO) Si H Si O (RO) 3 SiOSi(OR) 3
O
+ 3 + R
Si O
(RO) 3 R OR
H
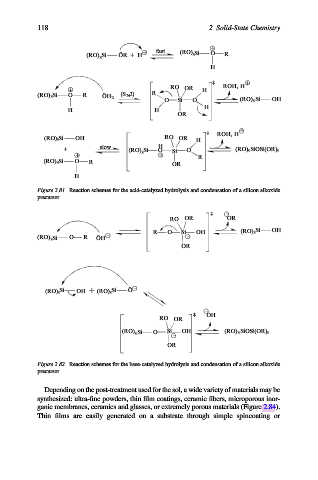
Figure 2.81. Reaction schemes for the acid-catalyzed hydrolysis and condensation of a silicon alkoxide
precursor.
‡ −
RO OR OR
R O Si OH (RO) 3 Si OH
Si O − −
(RO) 3 R ÖH
OR
(RO) Si OH + (RO) Si Ö −
3
3
−
‡ OH
RO OR
Si O Si OH (RO) SiOSi(OR)
(RO) 3 3 3
−
OR
Figure 2.82. Reaction schemes for the base-catalyzed hydrolysis and condensation of a silicon alkoxide
precursor.
Depending on the post-treatment used for the sol, a wide variety of materials may be
synthesized: ultra-fine powders, thin film coatings, ceramic fibers, microporous inor-
ganic membranes, ceramics and glasses, or extremely porous materials (Figure 2.84).
Thin films are easily generated on a substrate through simple spincoating or