Page 135 - Materials Chemistry, Second Edition
P. 135
122 2 Solid-State Chemistry
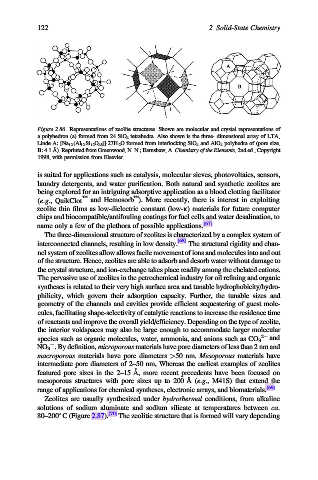
Figure 2.86. Representations of zeolite structures. Shown are molecular and crystal representations of
a polyhedron (a) formed from 24 SiO 4 tetrahedra. Also shown is the three- dimensional array of LTA,
Linde A: [Na 12 (Al 12 Si 12 O 48 )]·27H 2 O formed from interlocking SiO 4 and AlO 4 polyhedra of (pore size,
˚
B: 4.1 A). Reprinted from Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements, 2nd ed., Copyright
1998, with permission from Elsevier.
is suited for applications such as catalysis, molecular sieves, photovoltaics, sensors,
laundry detergents, and water purification. Both natural and synthetic zeolites are
being explored for an intriguing adsorptive application as a blood clotting facilitator
™
(e.g., QuikClot ™ and Hemosorb ). More recently, there is interest in exploiting
zeolite thin films as low-dielectric constant (low-k) materials for future computer
chips and biocompatible/antifouling coatings for fuel cells and water desalination, to
name only a few of the plethora of possible applications. [67]
The three-dimensional structure of zeolites is characterized by a complex system of
interconnected channels, resulting in low density. [68] The structural rigidity and chan-
nel system of zeolites allow allows facile movement of ions and molecules into and out
of the structure. Hence, zeolites are able to adsorb and desorb water without damage to
the crystal structure, and ion-exchange takes place readily among the chelated cations.
The pervasive use of zeolites in the petrochemical industry for oil refining and organic
syntheses is related to their very high surface area and tunable hydrophobicity/hydro-
philicity, which govern their adsorption capacity. Further, the tunable sizes and
geometry of the channels and cavities provide efficient sequestering of guest mole-
cules, facilitating shape-selectivity of catalytic reactions to increase the residence time
of reactants and improve the overall yield/efficiency. Depending on the type of zeolite,
the interior voidspaces may also be large enough to accommodate larger molecular
2
species such as organic molecules, water, ammonia, and anions such as CO 3 and
NO 3 . By definition, microporous materials have pore diameters of less than 2 nm and
macroporous materials have pore diameters >50 nm. Mesoporous materials have
intermediate pore diameters of 2–50 nm, Whereas the earliest examples of zeolites
˚
featured pore sizes in the 2–15 A, more recent precedents have been focused on
˚
mesoporous structures with pore sizes up to 200 A (e.g., M41S) that extend the
range of applications for chemical syntheses, electronic arrays, and biomaterials. [69]
Zeolites are usually synthesized under hydrothermal conditions, from alkaline
solutions of sodium aluminate and sodium silicate at temperatures between ca.
80–200 C (Figure 2.87). [70] The zeolitic structure that is formed will vary depending