Page 140 - Materials Chemistry, Second Edition
P. 140
127
2.4. The Amorphous State
4
6
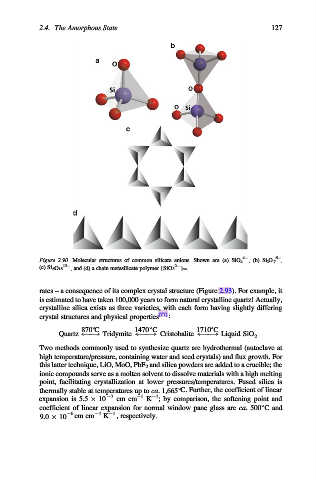
Figure 2.90. Molecular structures of common silicate anions. Shown are (a) SiO 4 , (b) Si 2 O 7 ,
12 2
(c) Si 6 O 18 , and (d) a chain metasilicate polymer {SiO 3 } 1.
rates – a consequence of its complex crystal structure (Figure 2.93). For example, it
is estimated to have taken 100,000 years to form natural crystalline quartz! Actually,
crystalline silica exists as three varieties, with each form having slightly differing
crystal structures and physical properties [77] :
870 C 1470 C 1710 C
Quartz ! Tridymite ! Cristobalite ! Liquid SiO 2
Two methods commonly used to synthesize quartz are hydrothermal (autoclave at
high temperature/pressure, containing water and seed crystals) and flux growth. For
this latter technique, LiO, MoO, PbF 2 and silica powders are added to a crucible; the
ionic compounds serve as a molten solvent to dissolve materials with a high melting
point, facilitating crystallization at lower pressures/temperatures. Fused silica is
thermally stable at temperatures up to ca. 1,665 C. Further, the coefficient of linear
1
expansion is 5.5 10 7 cm cm 1 K ; by comparison, the softening point and
coefficient of linear expansion for normal window pane glass are ca. 500 C and
1
9.0 10 6 cm cm 1 K , respectively.