Page 141 - Materials Chemistry, Second Edition
P. 141
128 2 Solid-State Chemistry
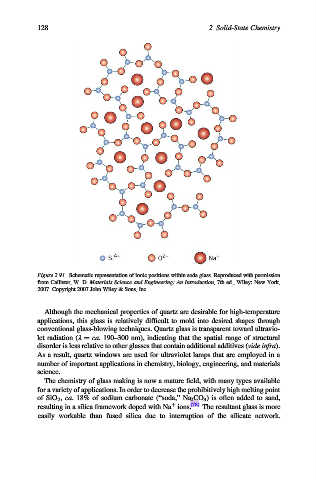
Figure 2.91. Schematic representation of ionic positions within soda glass. Reproduced with permission
from Callister, W. D. Materials Science and Engineering: An Introduction, 7th ed., Wiley: New York,
2007. Copyright 2007 John Wiley & Sons, Inc.
Although the mechanical properties of quartz are desirable for high-temperature
applications, this glass is relatively difficult to mold into desired shapes through
conventional glass-blowing techniques. Quartz glass is transparent toward ultravio-
let radiation (l ¼ ca. 190–300 nm), indicating that the spatial range of structural
disorder is less relative to other glasses that contain additional additives (vide infra).
As a result, quartz windows are used for ultraviolet lamps that are employed in a
number of important applications in chemistry, biology, engineering, and materials
science.
The chemistry of glass making is now a mature field, with many types available
for a variety of applications. In order to decrease the prohibitively high melting point
of SiO 2 , ca. 18% of sodium carbonate (“soda,” Na 2 CO 3 ) is often added to sand,
þ
resulting in a silica framework doped with Na ions. [78] The resultant glass is more
easily workable than fused silica due to interruption of the silicate network.